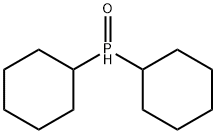
Dicyclohexylphosphine oxide synthesis
- Product Name:Dicyclohexylphosphine oxide
- CAS Number:14717-29-4
- Molecular formula:C12H23OP
- Molecular Weight:214.28
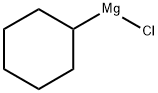
931-51-1
127 suppliers
$42.00/100ml
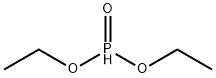
762-04-9
324 suppliers
$10.00/10 g

14717-29-4
40 suppliers
inquiry
Yield: 85%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at 0 - 25; for 16.5 h;Inert atmosphere;
Steps:
Synthesis of Dicyclohexylphosphine Oxide [14717-29-4].
Synthesis of Dicyclohexylphosphine Oxide [14717-29-4]. [00101] The compound was prepared according to a reported procedure. Under argon, a 1 -litre two-necked round bottom flask (RBF) was charged with magnesium turnings ( 13.2 g, 550 mmol) and 50 mL of dry diethyl ether. Then a crystal of iodine was added, followed by chlorocyclohexane (7.5 g, 63 mmol) over 5 min without stirring. After addition of another portion of 150 mL of dry diethyl ether, the rest of chlorocyclohexane (51.8 g, 437 mmol) in 25 mL of dry ether was added dropwise over ~ 30 min in such a rate that a gentle reflux was maintained. After completion of the addition, the reaction mixture was refluxed for another 45 o min. Then the mixture was diluted with 500 mL of dry THF, and cooled to 0 C in an ice bath. A solution of diethyl phosphate (21.0 g, 152 mmol) in 20 mL of dry THF was added into the o Grignard reagent dropwise over -10 min. After the addition, the mixture was stirred at 0 C for o another 30 min and then at 25 C for 16 h. At the end of the reaction, the mixture was cooled to O 0 0 C in an ice bath, and quenched with 0.1 N HC1 (380 mL). After stirring at 25 C for 5 min, the organic layer was separated and the insoluble salt was removed by filtering through a pad of CELITE with ethyl acetate washings (200 mL). The organic layer in the filtrate was separated and combined with the first organic layer, dried over MgS04, and concentrated on a rotary evaporator. The residue was purified by Kugelrohr distillation (fraction at -120 C at 1 300 mTorr) to give the target compound (27.6 g, 85%) as white solid. H NMR (400 MHz, CDC ): δ 6.31 (dt, ¾Ρ = 433.6 Hz, JHH = 2.8 Hz, 1H), 2.01 -1.22 (m, 22H). 3 p { 1H} NMR (162 MHz, CDCb): δ 49.4.
References:
NANYANG TECHNOLOGICAL UNIVERSITY;ZHOU, Steve, Jianrong;HUANG, Zhiyan WO2013/28132, 2013, A1 Location in patent:Paragraph 00101
16523-54-9
262 suppliers
$8.00/1g

14717-29-4
40 suppliers
inquiry
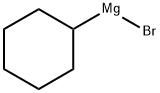
931-50-0
108 suppliers
$45.00/1g

14717-29-4
40 suppliers
inquiry
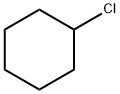
542-18-7
364 suppliers
$13.00/25g

14717-29-4
40 suppliers
inquiry