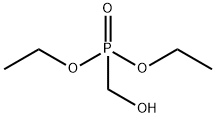
Diethyl (hydroxymethyl)phosphonate synthesis
- Product Name:Diethyl (hydroxymethyl)phosphonate
- CAS Number:3084-40-0
- Molecular formula:C5H13O4P
- Molecular Weight:168.13
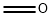
50-00-0
884 suppliers
$10.00/25g
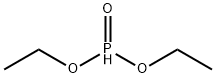
762-04-9
341 suppliers
$10.00/10 g

3084-40-0
254 suppliers
$10.00/10g
Yield:3084-40-0 96%
Reaction Conditions:
with triethylamine at 90; for 3 h;Reflux;
Steps:
O,O-Diethyl hydroxymethylphosphonate (10) [2]
Adapted from Phillion et al. To a stirred suspension of paraformaldehyde (2.22 g, 72 mmol, 1.00 equiv) in diethyl phosphite (11) (10.00 g, 72 mmol, 1.00 equiv) was added triethylamine (732 mg, 1.00 mL, 7 mmol, 0.10 equiv). The resulting suspension was heated to 90 °C and the suspension became clear and the mixture began reflux. The reaction was maintained at this temperature for three hours. The product was then purified by flash column chromatography (Rf 10 = 0.60, eluent - 100% EtOAC) and was isolated as a colourless oil (11.62 g, 70 mmol, 96%). 1H NMR (400 MHz, CDCl3): 1.35 (6H, t, J = 7.19 Hz, OCH2CH3), 3.91 (2H, dd appears as a triplet, JH-H = JH-P = 6.30 Hz, HOCH2P), 4.13-4.24 (4H, m, OCH2CH3), 4.81 - 4.86 (1H, m, HOCH2P). 13C NMR (100 MHz, CDCl3): 16.35 (d, J = 5.68 Hz, OCH2CH3), 56.87 (d, J = 162.21 Hz, HOCH2P), 62.51 (d, J = 6.76 Hz, OCH2CH3) 31P NMR (175 MHz, CDCl3): 24.52. HRMS (ESI+): Exact mass calc. C5H14O4P [M+H] = 169.0627; Found = 169.0630.
References:
Jones, David J.;O’Leary, Eileen M.;O’Sullivan, Timothy P. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 801 - 810] Location in patent:supporting information
![Phosphonic acid, [(acetyloxy)methyl]-, diethyl ester (9CI)](/CAS/20210111/GIF/7016-78-6.gif)
7016-78-6
0 suppliers
inquiry

3084-40-0
254 suppliers
$10.00/10g
110-71-4
494 suppliers
$13.00/25ml

762-04-9
341 suppliers
$10.00/10 g

3084-40-0
254 suppliers
$10.00/10g
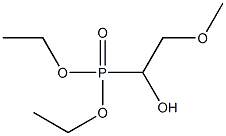
19462-41-0
2 suppliers
inquiry
