
Doripenem synthesis
1synthesis methods
- Product Name:Doripenem
- CAS Number:148016-81-3
- Molecular formula:C15H24N4O6S2
- Molecular Weight:420.5
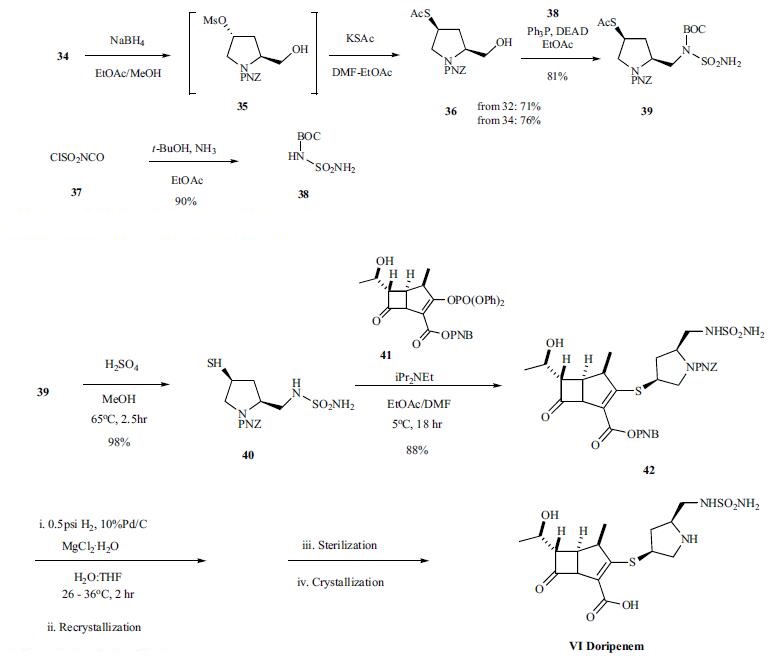
the hydroxyl proline is protected as the PNZ ester 32 first in
95% yield. The protected proline acid 32 was converted to
the methyl ester with refluxing sulfuric acid in methanol
followed by conversion of the alcohol to the mesylate 34 in
91% overall yield from 30. The mesylate ester was reduced
with sodium borohydride to provide alcohol 35, which was
converted without purification to thiol ester 36 by reacting
with potassium thioacetate. Mitsunobu reaction
of alcohol 36 with BOC-sulfonyl urea 38, which was prepared
from chlorosulfonyl isocyanate with ammonia in tbutanol
in 90% yield, provided the key thioacetate intermediate
39. Finally, protected doripenem 42 was prepared by
coupling thiol 40, obtained by hyrolysis of thioacetate 39,
with enolphosphate 41 in 88% yield. Deprotection
of intermediate ester and carbamate protecting groups
via hydrogenation gave the desired carbapenem VI, which
was isolated after crystallization. Final form of the drug
doripenem was prepared by sterilization, crystallization and
granulation.
![(4R,5S,6S)-3-[[(3S,5S)-5-[[(Aminosulfonyl)amino]methyl]-1-[[(4-nitrophenyl)methoxy]carbonyl]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (4-nitrophenyl)methyl ester](/CAS2/GIF/491878-07-0.gif)