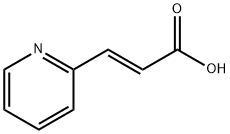
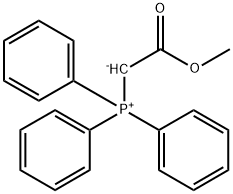
(E)-Methyl 3-(pyridin-2-yl)acrylate synthesis
- Product Name:(E)-Methyl 3-(pyridin-2-yl)acrylate
- CAS Number:81124-45-0
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
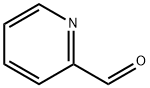
1121-60-4
508 suppliers
$10.60/10gm:
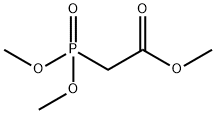
5927-18-4
322 suppliers
$5.00/25g

81124-45-0
8 suppliers
inquiry
Yield:81124-45-0 174 mg
Reaction Conditions:
in tetrahydrofuran;hexane; for 1 h;Cooling with ice;Horner-Wadsworth-Emmons Olefination;
Steps:
Typical experimental procedure for one-pot preparation of aryl or heteroaryl-bearing acrylates (6).Methyl (E)-3-(pyridin-2-yl)acrylate (6d):11 (Table 2, entry 4)
To a solution of 5d (249 mg, 1.58 mmol) in THF (2 mL) was added n-BuLi (2.2 M in hexane, 0.86 mL,1.89 mmol) at -25 °C with stirring for 30 min. To the solution was added dropwise DMF (0.15 mL, 1.9mmol) with stirring at that temperature for 40 min, then allowed to warmed to ice-bath temperature andstirred for additional 40 min. Trimethyl phosphonoacetate (0.30 mL, 2.1 mmol) was added with stirring atice-bath temperature and the stirring was continued for 1 h. Aqueous 5% ammonium acetate (8 mL) wasadded and the mixture was extracted with EtOAc (20 mL). The organic layer was washed with 5%sodium acetate aq. (8 mL x 2), dried over anhydrous sodium sulfate, and concentrated. The residual oilwas subjected to column chromatography (silica gel) to give 6d (174 mg, 68%) as a yellow oil. 1H NMR δ 8.66 (1H, d, J = 4 Hz), 7.73-7.67 (1H, m), 7.69 (1H, d, J = 16 Hz), 7.42 (1H, d, J = 8 Hz),7.28-7.25 (1H, m), 6.93 (1H, d, J = 16 Hz), 3.82 (3H, s); 13C NMR δ 167.23, 152.95, 150.19, 143.57,136.76, 124.25, 124.23, 122.00, 51.84; MS m/z 164 ([M+H]+).
References:
Yasukata, Tatsuro;Matsuura, Takaharu [Heterocycles,2021,vol. 102,# 3,p. 527 - 533]

1121-60-4
508 suppliers
$10.60/10gm:
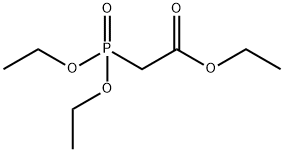
867-13-0
462 suppliers
$10.00/1g

81124-45-0
8 suppliers
inquiry