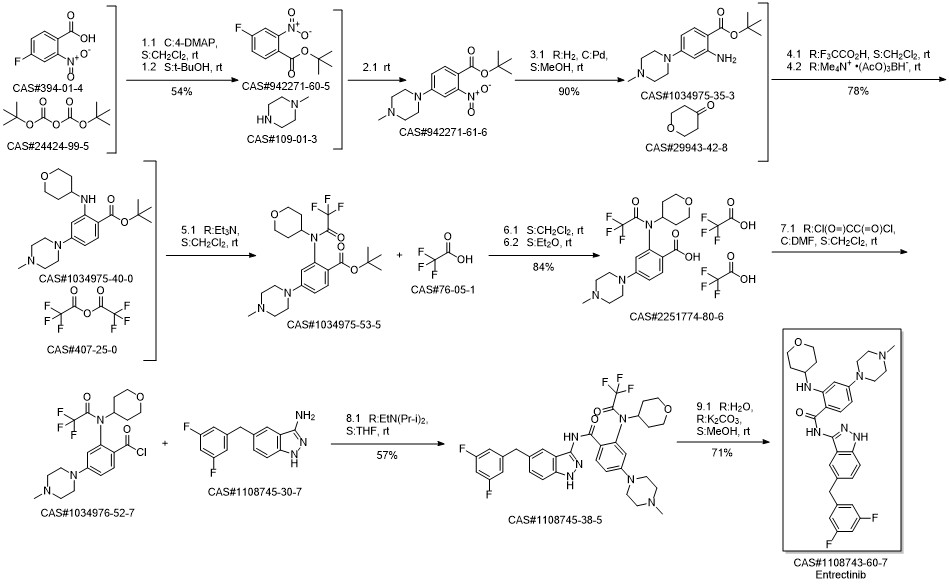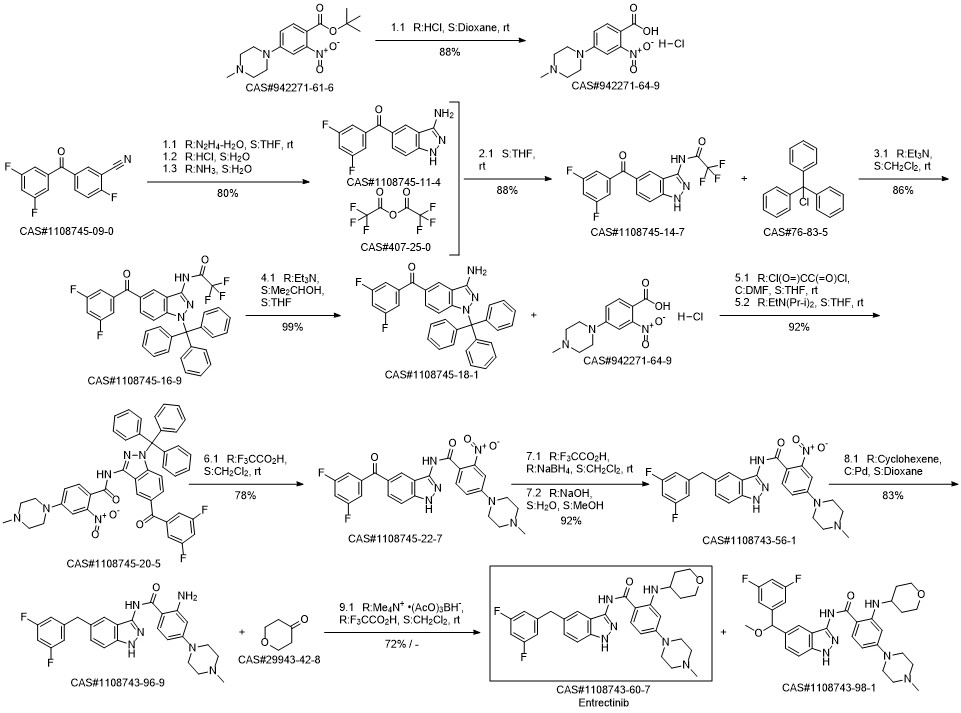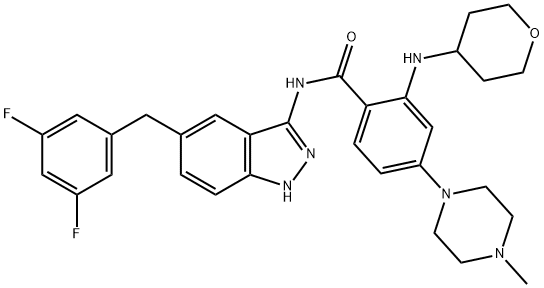
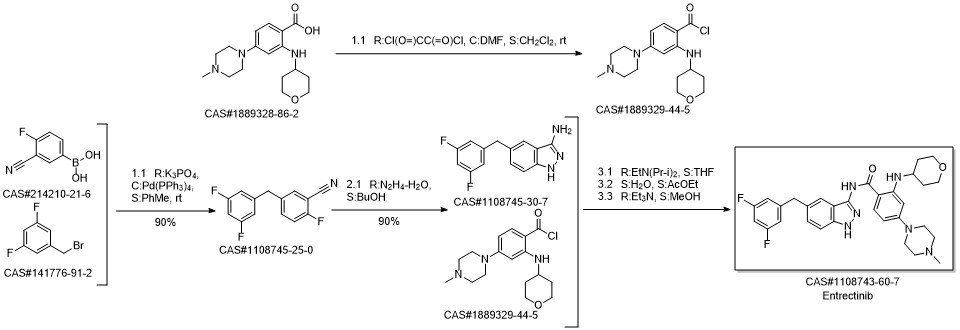
Entrectinib synthesis
- Product Name:Entrectinib
- CAS Number:1108743-60-7
- Molecular formula:C31H34F2N6O2
- Molecular Weight:560.64
Reference: Menichincheri, Maria; Ardini, Elena; Magnaghi, Paola; Avanzi, Nilla; Banfi, Patrizia; Bossi, Roberto; Buffa, Laura; Canevari, Giulia; Ceriani, Lucio; Colombo, Maristella; Corti, Luca; Donati, Daniele; Fasolini, Marina; Felder, Eduard; Fiorelli, Claudio; Fiorentini, Francesco; Galvani, Arturo; Isacchi, Antonella; Borgia, Andrea Lombardi; Marchionni, Chiara; Nesi, Marcella; Orrenius, Christian; Panzeri, Achille; Pesenti, Enrico; Rusconi, Luisa; Saccardo, Maria Beatrice; Vanotti, Ermes; Perrone, Ettore; Orsini, Paolo. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. Journal of Medicinal Chemistry. Volume 59. Issue 7. Pages 3392-3408. Journal; Online Computer File. (2016).
![Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)(2,2,2-trifluoroacetyl)amino]-](/CAS/20200611/GIF/1108745-38-5.gif)
1108745-38-5
11 suppliers
inquiry

1108743-60-7
196 suppliers
$28.00/1mg
Yield:1108743-60-7 78%
Reaction Conditions:
with triethylamine in methanol at 65; for 2 h;Product distribution / selectivity;
Steps:
2.i'
Alternatively, not previously purified crude reaction mixture can be dissolved in methanol (375 mL) in the presence of triethylamine (60 mL) and stirred at 65°C for 2 hours. The solvents were removed under reduced pressure and the residue treated with water / ethyl acetate. Organic phase was dried over sodium sulfate and evaporated to dryness. Purification of the crude by chromatography over silica gel (DCM/EtOH/NH3 5N in MeOH = 1000/50/5) and crystallisation of the so obtained compound from EtOAc / hexane afforded 8.4 g of the title compound as a white solid (78% yield). IH-NMR (400 MHz), δ (ppm, DMSO-d6): 1.26 - 1.43 (m, 2H) 1.86 - 2.02 (m, 2H) 2.23 (s, 3H) 2.42 - 2.46 (m, 4H) 3.23 - 3.29 (m, 4H) 3.45 - 3.54 (m, 2H) 3.62 - 3.75 (m, IH) 3.82 (dt, J=I 1.61, 3.83 Hz, 2H) 4.05 (s, 2H) 6.14 (d, J=2.07 Hz, IH) 6.24 (dd, J=8.90, 2.19 Hz, IH) 6.94 - 7.06 (m, 3H) 7.26 (dd, J=8.66, 1.46 Hz, IH) 7.41 (d, J=8.66 Hz, IH) 7.50 (d, IH) 7.80 (d, J=9.15 Hz, IH) 8.29 (d, J=7.68 Hz, IH) 10.08 (s, IH) 12.63 (s, IH).
References:
NERVIANO MEDICAL SCIENCES S.R.L. WO2009/13126, 2009, A1 Location in patent:Page/Page column 76
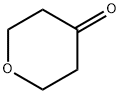
29943-42-8
432 suppliers
$5.00/1g
![Benzamide, 2-?amino-?N-?[5-?[(3,?5-?difluorophenyl)?methyl]?-?1H-?indazol-?3-?yl]?-?4-?(4-?methyl-?1-?piperazinyl)?-](/CAS/20200611/GIF/1108743-96-9.gif)
1108743-96-9
2 suppliers
inquiry

1108743-60-7
196 suppliers
$28.00/1mg
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid trifluoroacetate](/CAS/20180703/GIF/1034975-62-6.gif)
1034975-62-6
53 suppliers
inquiry

1108743-60-7
196 suppliers
$28.00/1mg

214210-21-6
225 suppliers
$17.00/250mg

1108743-60-7
196 suppliers
$28.00/1mg

141776-91-2
251 suppliers
$5.00/1g

1108743-60-7
196 suppliers
$28.00/1mg