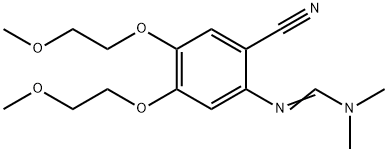
Erlotinib Impurity 22 synthesis
- Product Name:Erlotinib Impurity 22
- CAS Number:950596-59-5
- Molecular formula:C16H23N3O4
- Molecular Weight:321.37
950596-58-4
141 suppliers
inquiry
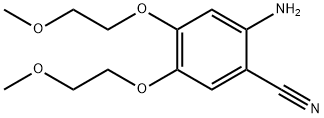
4637-24-5
589 suppliers
$5.00/25g

950596-59-5
21 suppliers
inquiry
Yield:950596-59-5 88%
Reaction Conditions:
with acetic acid in toluene at 80 - 90;
Steps:
1 Example 1 Preparation of N'-[2-Cyano-4,5-bis(2-methoxyethoxy)phenyl]-N,N-dimethyl-formamidine
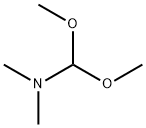
2-amino-4,5-bis(2-methoxyethoxy)benzonitrile) 70 g, DMF-DMA (N,N-dimethylformamide dimethyl acetal) 34.5 g, toluene 350 mL, The mixture of glacial acetic acid 4mL, mechanical stirring, heated to 80 ~ 90 °C reaction 3 ~ 4h, the reaction is completed, After concentration under reduced pressure, ethyl acetate (60 mL) was added to the concentrated oil, and methyl tert-butyl ether (100 mL) was added. After the solution is heated and clarified, it is cooled and crystallized, that is, it is stirred at a temperature of 15-20°C for 2 hours. Then at a temperature of 0 ~ 5 °C stirring 1h, suction filtration to obtain a solid at a temperature of 20 ~ 25 °C drying under reduced pressure 4 ~ 5h, The compound of formula II is obtained in 73 g in a yield of 88%.
References:
CN105001166,2018,B Location in patent:Paragraph 0034; 0036
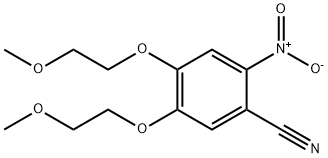
236750-65-5
169 suppliers
inquiry

950596-59-5
21 suppliers
inquiry

950596-58-4
141 suppliers
inquiry

950596-59-5
21 suppliers
inquiry

80407-68-7
53 suppliers
inquiry

950596-59-5
21 suppliers
inquiry
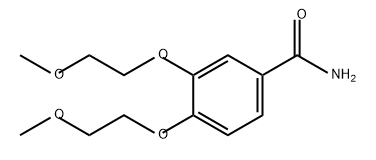
1418289-91-4
0 suppliers
inquiry

950596-59-5
21 suppliers
inquiry