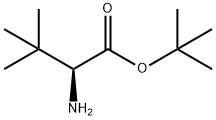
H-TBU-GLY-OTBU HCL synthesis
- Product Name:H-TBU-GLY-OTBU HCL
- CAS Number:31556-74-8
- Molecular formula:C10H21NO2
- Molecular Weight:187.28
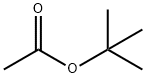
540-88-5
391 suppliers
$21.00/25mL

20859-02-3
623 suppliers
$9.00/5 g

31556-74-8
35 suppliers
$75.00/250 mg
Yield:-
Reaction Conditions:
with perchloric acid in ethyl acetate
Steps:
Stage 1c: (2S)-2-Amino-3,3-dimethyl-butanoic acid tert-butyl ester (456)
Stage 1c: (2S)-2-Amino-3,3-dimethyl-butanoic acid tert-butyl ester (456) Tert-Leucine (1.5 g, 11.43 mmol, 1.0 eq.) and tert-butyl acetate (30 mL) were charged into a 100 mL round bottom flask and the reaction mixture cooled to 0° C. Perchloric acid (1.72 g, 1 mL, 17.2 mmol, 1.5 eq.) was added dropwise and the reaction mixture was left to warm up to ambient temperature and stirred for a further 48 hours. The organic phase was washed with water (50 mL) and then 1M hydrochloric acid (30 mL). The aqueous phases were combined and the pH adjusted to 9 with 1M aqueous potassium carbonate solution. The aqueous phase was extracted with dichloromethane (3*40 mL). The first organic phase and the dichloromethane extracts were combined, dried over sodium sulfate, filtered and the solvent removed under vacuum (caution: desired product has a low boiling point keep Buchi batch cold and pressure around 100 mbars). The residue was purified by flash column chromatography, using ethyl acetate:heptanes (1:1) as eluent. After combining the relevant fractions and solvent removal, 1.20 g (56%) of the title compound was isolated as a colorless oil. 1H NMR (250 MHz, CHLOROFORM-d) δ ppm 3.03 (s, 1H) 1.56 (s, 2H) 1.48 (s, 9H) 0.97 (s, 9H)
References:
INTERMUNE, INC. US2010/119479, 2010, A1

20859-02-3
623 suppliers
$9.00/5 g
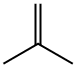
115-11-7
245 suppliers
$45.00/25 g

31556-74-8
35 suppliers
$75.00/250 mg
![L-Valine, 3-methyl-N-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/31556-73-7.gif)
31556-73-7
0 suppliers
inquiry

31556-74-8
35 suppliers
$75.00/250 mg

20859-02-3
623 suppliers
$9.00/5 g

31556-74-8
35 suppliers
$75.00/250 mg
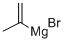
13291-18-4
174 suppliers
$82.59/100ml
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

74-88-4
360 suppliers
$15.00/10g

31556-74-8
35 suppliers
$75.00/250 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry