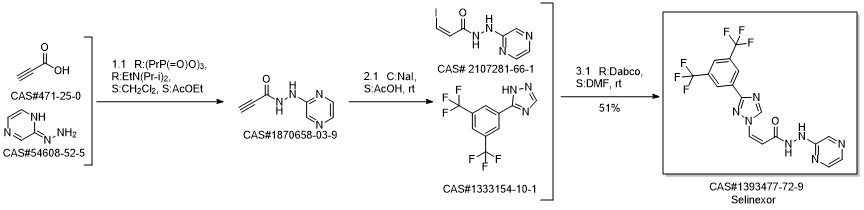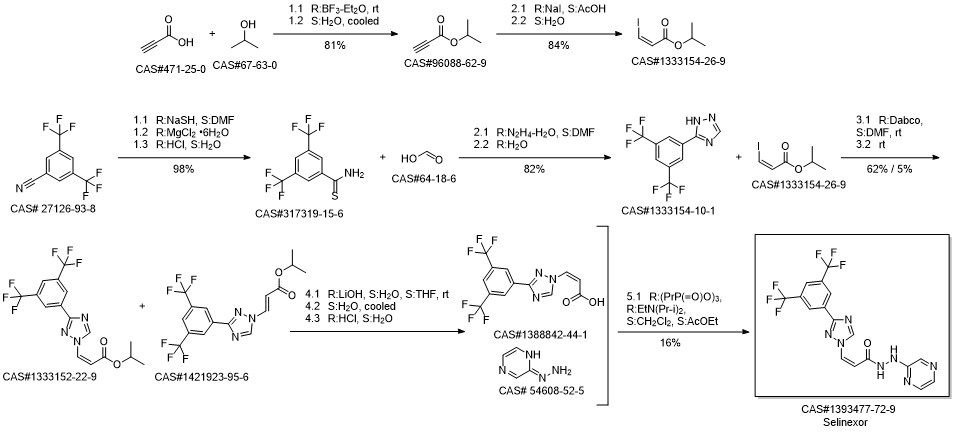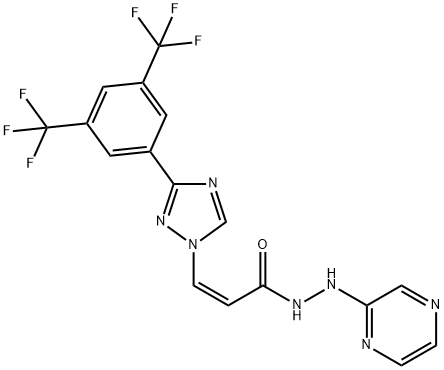
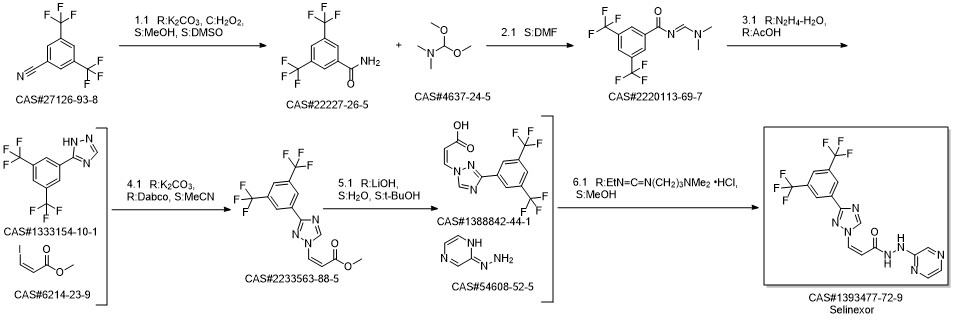
KPT-330 synthesis
- Product Name:KPT-330
- CAS Number:1393477-72-9
- Molecular formula:C17H11F6N7O
- Molecular Weight:443.31
Reference: Muthusamy, Anantha Rajmohan; Kanniah, Sundara Lakshmi; Ravi, Akash; Das, Tonmoy Chitta; Chemate, Rajendra Popat; Singh, Anil Kumar; Wagh, Yogesh Dhananjay. Novel crystalline forms of selinexor and process for their preparation. Assignee Watson Laboratories Inc., USA. WO 2018129227. (2018).
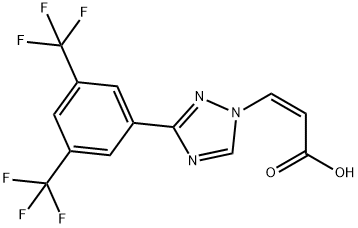
1388842-44-1
40 suppliers
inquiry
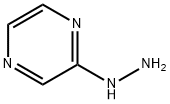
54608-52-5
226 suppliers
$9.00/1g

1393477-72-9
138 suppliers
$29.00/1mg
Yield:1393477-72-9 83%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in ethyl acetate;acetonitrile at 0 - 25; for 3 h;Solvent;Temperature;
Steps:
28 Example 28: Preparation of Selinexor
In a 3-L, 3-necked, round-bottomed flask were charged 60.0 gr (Z)-3-(3-(3,5- bis(trifluoromethyl)phenyl)- 1 H- 1,2, 4-triazol- 1 -yl)acrylic acid (SLN- 105, prepared according to examples 27), Ethyl acetate (0.42 lit, 7V) and Acetonitrile (0.3 lit,5V) at 20-25°C. Charged 2-hydrazino pyrazine (19.8 gr, 1.05 eq) then cooled to 0 to 5°C. Charged EDC .HC1 (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) (49. lgr 1 .Seq) at 0 to 5°C. The reaction mass was stirred for 3hrs and monitored by HPLC (till SLN-105 NMT 1.0%). Once the reaction completes, charge water (0.2lit, 2V) and stirred for 15-30 mm at 15-20°C, settled and separated the organic layer. Collected the organic layer and washed with sodium bicarbonate solution (0.Slit, SV). Finally washed the organic layer with water (0.2lit, 2 V) and combined the collected organic layer containing the product. The solvent is distilled off under vacuum at 50 to 60°C for 30 mm. To the obtained solid, added absolute Ethanol (0.6lit, 1OV) and stirred for 30mm at 20-25°C then cooled to 0-5°C and stirred for 1 hr at 0-5°C. Filtered the compound under vacuum at 20-25°C and washed with Ethanol (0.2lit, 2V). The wet cake was dried at 55-60°C under vacuum (600 to 700 mm Hg) for 4 hrs. (Yield 83%).
References:
WATSON LABORATORIES INC.;MUTHUSAMY, Anantha Rajmohan;KANNIAH, Sundara Lakshmi;RAVI, Akash;DAS, Tonmoy Chitta;CHEMATE, Rajendra Popat;SINGH, Anil Kumar;WAGH, Yogesh Dhananjay WO2018/129227, 2018, A1 Location in patent:Paragraph 00196; 00197; 00215; 00216; 00218
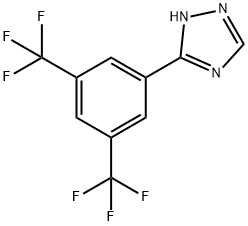
1333154-10-1
59 suppliers
inquiry

1393477-72-9
138 suppliers
$29.00/1mg
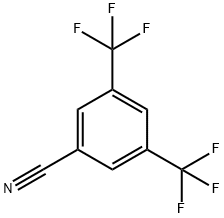
27126-93-8
277 suppliers
$6.00/1g

1393477-72-9
138 suppliers
$29.00/1mg

317319-15-6
46 suppliers
$51.39/250mg

1393477-72-9
138 suppliers
$29.00/1mg
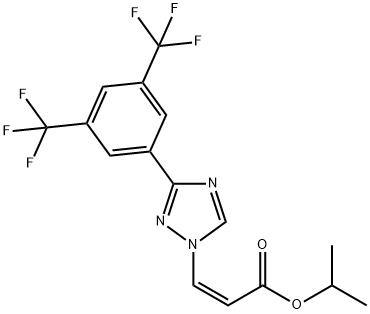
1333152-22-9
21 suppliers
inquiry

1393477-72-9
138 suppliers
$29.00/1mg