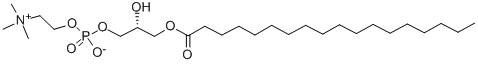
L-ALPHA-LYSOPHOSPHATIDYLCHOLINE, STEAROYL synthesis
- Product Name:L-ALPHA-LYSOPHOSPHATIDYLCHOLINE, STEAROYL
- CAS Number:19420-57-6
- Molecular formula:C26H54NO7P
- Molecular Weight:523.68
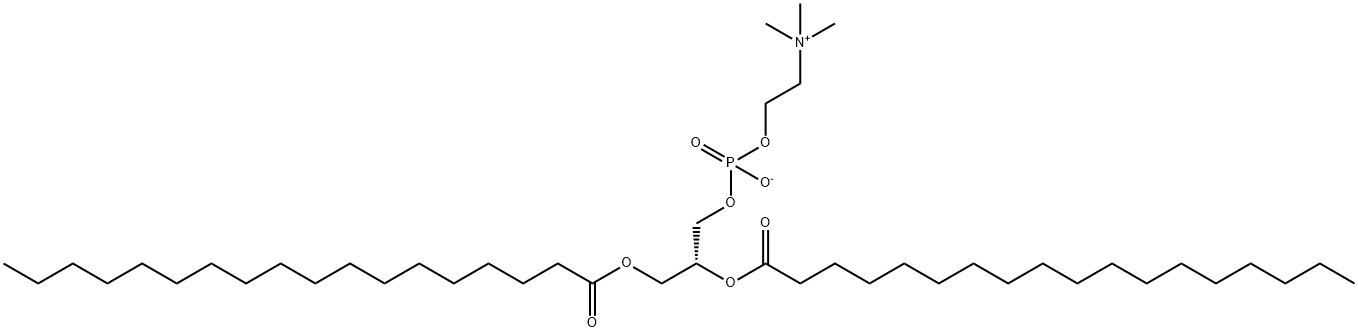
816-94-4
256 suppliers
$28.00/250mg

19420-57-6
143 suppliers
$54.00/100mg
Yield:19420-57-6 99%
Reaction Conditions:
with tris hydrochloride;sodium docusate;calcium chloride in 2,2,4-trimethylpentane at 40; pH=8.5; for 0.5 h;Enzymatic reaction;
Steps:
General Procedure for 1,2-Diacyl-sn-glycero-3-phosphocholines Enzymatic Hydrolysis
General procedure: Hydrolysis of 1,2-diacyl-sn-glycero-3-phosphocholines 2a-e was carried out according to the method described by Morgado et al.[30] Two solutions, A: 0.635 mmol of 2a-e in 13.2 mL isooctane, and B: 174 mg (0.393 mmol) of sodium docusate (AOT) in 13.2mL isooctane, were pre-incubated for 0.5 h at 40 °C on a magnetic stirrer. Then, 262 mL of PLA2 (10000 U mL-1; 1 U is equivalent to the amount of enzyme producing 1 mmole of free fatty acid per minute at pH 8 and 40 °C) and the same volume of Tris-HCl buffer (0.1 M, pH 8.5) with 0.75M of CaCl2 were then added to solution B and the reaction was started by adding solution A. The progress of the enzymatic reactions was monitored by TLC (65 : 25 : 4 CHCl3/CH3OH/H2O v/v/v). The reaction was carried out for 30 min. The enzyme was separated from the reaction mixture using diatomaceous earth (Celite 545) and the solvent was evaporated to dryness at 40 °Cunder vacuum. 1-Acyl LPCs 3a-e were purified on a silica gelcolumn (65 : 25 : 4 CHCl3/CH3OH/H2O v/v/v). Product containing fractions of Rf 0.3 were collected and solvent evaporated under vacuum (45 °C).
References:
Niezgoda, Natalia;Gliszczyńska, Anna;G?adkowski, Witold;Kempińska, Katarzyna;Wietrzyk, Joanna;Wawrzeńczyk, Czes?aw [Australian Journal of Chemistry,2015,vol. 68,# 7,p. 1065 - 1075]
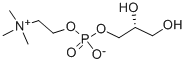
28319-77-9
475 suppliers
$5.00/250mg
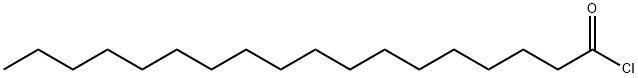
112-76-5
247 suppliers
$40.32/25G

19420-57-6
143 suppliers
$54.00/100mg
![Ethanaminium, 2-[[hydroxy[(2R)-3-[(1-oxooctadecyl)oxy]-2-(phenylmethoxy)propoxy]phosphinyl]oxy]-N,N,N-trimethyl-, inner salt](/CAS/20211123/GIF/171597-35-6.gif)
171597-35-6
0 suppliers
inquiry

19420-57-6
143 suppliers
$54.00/100mg
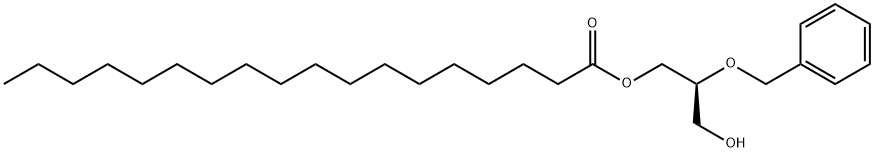
85647-89-8
0 suppliers
inquiry

19420-57-6
143 suppliers
$54.00/100mg
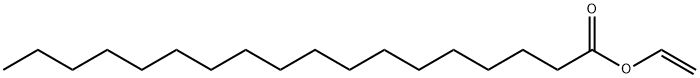
111-63-7
80 suppliers
$27.00/5g

19420-57-6
143 suppliers
$54.00/100mg