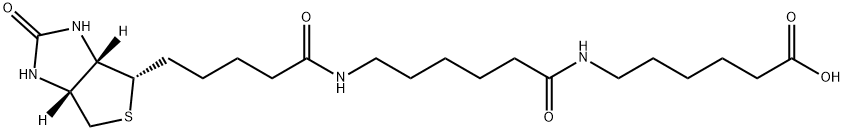
LC-LC(+)-Biotin synthesis
- Product Name:LC-LC(+)-Biotin
- CAS Number:89889-51-0
- Molecular formula:C22H38N4O5S
- Molecular Weight:470.6259
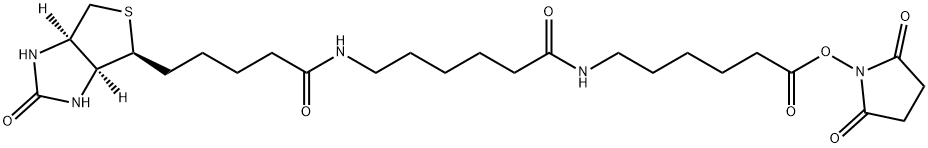
89889-52-1
108 suppliers
$20.00/5MG
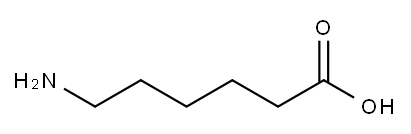
60-32-2
589 suppliers
$5.00/10g

89889-51-0
69 suppliers
$69.00/100mg
Yield: 72.2%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide
Steps:
3.2. General procedure for the synthesis of biotin derivatives
General procedure: A solution of Biotin (488 mg, 2.0 mmol, 1.0 equiv) in N, N-dimethylformamide(DMF, 20 ml) was added N-hydroxysuccinimide (NHS, 276 mg, 2.4 mmol, 1.2 equiv) and dicyclohexylcarbodiimide (DCC, 824 mg, 4.0 mmol, 2.0 equiv) and 4-dimethylaminopyridine(DMAP, 610 mg, 5.0 mmol, 2.5 equiv) at room temperature. The solution was stirred overnight, and then the solvent was removed under reduced pressure. The residue was dissolved in isopropanol (40 ml) with ultrasonic. The solution was cool to 4 C and stood for 1 h. The resulted mixture was filtered to afford intermediate a. Intermediate a (341 mg, 1.0 mmol, 1.0 equiv) and 6-aminocaproic acid(157 mg, 1.2 mmol, 1.2 equiv) were dissolved in 20 ml DMF. Triethylamine (0.3 ml,2.16 mmol, 2.16 equiv) was added. The solution was stirred overnight, and then the solvent was removed under reduced pressure. The residue was dissolved in water(25 ml) and formic acid (2 ml) with ultrasonic. The solution was cooled to 4 C. The resulted mixture was filtered to afford compound 1. Intermediate b was obtained according to the similar procedure of preparing intermediate a staring from 1. Compound 2 was obtained according to the similar procedure of preparing compound 1 staring from intermediate b. To a solution of biotin (400 mg, 2.0 mmol, 1.0equiv) and tributylamine (0.64 ml, 2.7 mmol, 1.3 equiv) in DMF (40 ml) was added isobutyl chloroformate (0.32 ml, 2.5 mmol, 1.2 equiv) at room temperature. The reaction mixture was stirred 10 min, and then 3-aminobenzoic acid (548 mg, 4.0 mmol,2.0 equiv) in 40 ml DMF was added slowly to the mixture at 0 C and left to stir for 2 h. The solvent was removed under reduced pressure. The residue was dissolved inwarm ethanol solution (50%, 36 ml). The pH of the mixture was adjusted to 2.0 by hydrochloric acid. The mixture was cooled to 0 C and stood for 12 h. The resulted mixture was filtered to give compound 3. Compounds 4a, 4 b and 4c were obtained according to the similar procedure of preparing 3 starting from 4-aminobenzoic acid, 4-amino-3-methylbenzoic acid and 4-amino-3-fluorobenzoic acid. The biotin derivatives were all white solids. Compound 1, 270 mg, yield 75.6%. Compound 2, 340 mg,yield 72.2%. Compound 3, 465 mg, yield 62.4%. Compound 4a, 483 mg, yield 64.9%. Compound 4b, 600 mg, yield 77.6%. Compound 4c, 465 mg, yield 62.4%.
References:
Huang, Xiao-Lei;Chen, Jing-Lei;Li, Xian-Lun;Zhao, Lei;Cui, Ya-Dong;Liu, Jiang-Yun;Morris-Natschke, Susan L.;Masuo, Goto;Cheng, Yung-Yi;Lee, Kuo-Hsiung;Chen, Dao-Feng;Zhang, Jian [Journal of Asian Natural Products Research,2021,vol. 23,# 7,p. 703 - 711]
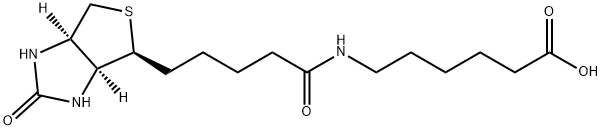
72040-64-3
137 suppliers
$25.00/100mg

60-32-2
589 suppliers
$5.00/10g

89889-51-0
69 suppliers
$69.00/100mg

58-85-5
976 suppliers
$5.00/10mg

89889-51-0
69 suppliers
$69.00/100mg
35013-72-0
350 suppliers
$12.00/100mg

89889-51-0
69 suppliers
$69.00/100mg

72040-64-3
137 suppliers
$25.00/100mg

89889-51-0
69 suppliers
$69.00/100mg