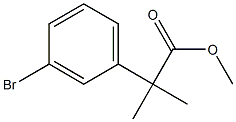
Methyl 2-(3-broMophenyl)-2-Methylpropanoate synthesis
- Product Name:Methyl 2-(3-broMophenyl)-2-Methylpropanoate
- CAS Number:251458-15-8
- Molecular formula:C11H13BrO2
- Molecular Weight:257.12
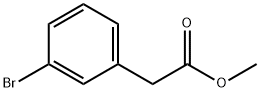
150529-73-0
150 suppliers
$6.00/1g

74-88-4
340 suppliers
$15.00/10g

251458-15-8
43 suppliers
$131.00/1g
Yield: 67%
Reaction Conditions:
Stage #1:methyl 2-(3-bromophenyl)acetatee with sodium hydride in tetrahydrofuran;mineral oil at 50; for 2 h;Inert atmosphere;
Stage #2:methyl iodide in tetrahydrofuran;mineral oil at 20 - 40;
Steps:
Sodium hydride (60% in oil) (10.4 g, 436 mmol) was added to tetrahydrofuran (400 ml) under argon and heated with stirring to 50° C. Methyl 2-(3-bromophenyl)acetate (20 g, 87.3 mmol) was added drop wise over 30 minutes and heating continued for 90 minutes. The temperature was lowered to below 40° C. and methyl iodide (13 ml, 209 mmol) was added over 10 minutes. The resulting suspension was stirred at room temperature overnight. Water (300 ml) was carefully added and reaction mixture concentrated. Residue was partitioned between diethyl ether (400 ml) and water. The aqueous layer was extracted with diethyl ether (400 ml), and the combined ethereal extracts were dried over sodium sulfate and concentrated. Crude oil was purified by column chromatography, eluding with a gradient from 0% to 20% ethyl acetate in heptane to give methyl 2-(3-bromophenyl)-2-methylpropanoate (14.96 g, 58 mmol, 67%). 1H NMR (400 MHz, CDCl3): δ ppm 7.47 (t, 1H), 7.37 (dt, 1H), 7.24 (dt, 1H), 7.18 (t, 1H), 3.65 (s, 3H), 1.55 (s, 6H)
References:
pfizer Inc US2010/197591, 2010, A1 Location in patent:Page/Page column 15-16

74-88-4
340 suppliers
$15.00/10g

251458-15-8
43 suppliers
$131.00/1g
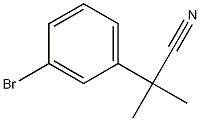
90433-20-8
40 suppliers
inquiry

251458-15-8
43 suppliers
$131.00/1g