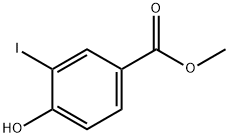
METHYL 4-HYDROXY-3-IODOBENZOATE synthesis
- Product Name:METHYL 4-HYDROXY-3-IODOBENZOATE
- CAS Number:15126-06-4
- Molecular formula:C8H7IO3
- Molecular Weight:278.04
99-76-3
751 suppliers
$5.00/25g

15126-06-4
135 suppliers
$6.00/250mg
Yield: 90.3%
Reaction Conditions:
with Iodine monochloride in acetic acid at 20 - 65; for 21.6667 h;
Steps:
68
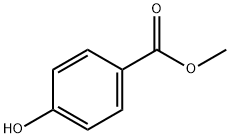
Methyl 4-hydroxybenzoate (35.5g, 0.233 mol) was dissolved in 200 mL of acetic acid, and the stirred mixture was warmed to 65°C. A solution of ICI (37.8 g, 0.233 mol) in 50 mL of AcOH was added dropwise over 40 min. The mixture was stirred at 65°C for 5 h and then stirred an additional 16 h at room temperature. The precipitated product was isolated via filtration, washed with water and dried under vacuum to give 27.5 g (99% pure by LCMS and HNMR) ) of desired product. The mother liquors were evaporated and resulting residue was washed with water and dried under vacuum to give another 31 g (95% pure by LCMS and NMR) of desired product. The combined yield of methyl 4-hydroxy-3-iodobenzoate was 58.5 g (90.3% yield). LCMS m/z [00438] Methyl 3-cyano-4-hydroxybenzoate 3: 28 g (0.1 mol) of methyl 4-hydroxy-3- iodobenzoate 2 dissolved in 100 mL of DMF was treated with 9.92 g (0.11 mol) of CuCN and 0.49 g (0.11 mol) of NaCN. The system was flushed with nitrogen after which the mixture warmed to 105°C and stirred to 18 h. The mixture was allowed to cool to room temperature, and any precipitates were removed via filtration and washed with EtOAc. The combined organics were diluted with 200 mL of water and then extracted with EtOAc (2x200 mL). The combined layers were dried over sodium sulfate, filtered and evaporated to dryness. After drying under vacuum, the resulting 18 g (100% yield) of 3 was characterized by LCMS and HNMR. [00439] Methyl 3-cyano-4-isopropoxybenzoate 4: Methyl 3-cyano-4-hydroxybenzoate 3 (18 g, 0.1 I mol) was dissolved in 100 mL of DMF and treated with 14.2 mL (0.15 mol) of 2- bromopropane and 41,9 g (0.3 mol) of anhydrous potassium carbonate. The system was flushed with nitrogen, and the mixture was heated to 90°C and stirred ovemight. After cooling to room temperature, the mixture was diluted with 200 mL of water and extracted with CH2CI2 (2x200 mL). The combined organic layers were dried over sodium sulfate, filtered and evaporated to dryness to give 20.5 g (99% yield) of 4 as an oil that was characterized by LCMS and HNMR. [00440] Perfluorophenyl 3-cyano-4-isopropoxybenzoate 6: 20.5 g (0.093 mol) of methyl 3-cyano-4-isopropoxybenzoate 4 was dissolved in 200 mL of a 6: 4 mixture of methanol and water. To this was added 5.61 g (0.14 mol) of NaOH, and the mixture was stirred for 2 hours at room temperature. The solution was then filtered through a silica gel plug and the solvents removed under vacuum. The resulting solid was re-dissolved in 200 mL of CH2Cl2 and treated with 19.3 mL (0.11 mol) of perfluorophenyl 2,?,2-trifluoroacetate 5 and 19.5 mL (0.14 mol) of triethylamine. After stining overnight, the solution was filtered and any solids rinsed with CH2Cl2. The combined organic mixtures were run through a short silica gel column and then evaporated to dryness to give 29 g (83.5% yield) of 6 which was characterized by LCMS and HNMR
References:
CYTOKINETICS, INC.;SMITHKLINE BEECHAM CORPORATION WO2005/107762, 2005, A2 Location in patent:Page/Page column 253-254

67-56-1
739 suppliers
$7.29/5ml-f

37470-46-5
103 suppliers
$22.00/100mg

15126-06-4
135 suppliers
$6.00/250mg

37470-46-5
103 suppliers
$22.00/100mg

74-88-4
342 suppliers
$15.00/10g

15126-06-4
135 suppliers
$6.00/250mg
3337-66-4
64 suppliers
inquiry

15126-06-4
135 suppliers
$6.00/250mg