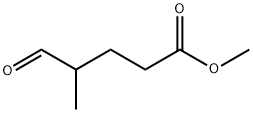
Methyl 4-Methyl-5-oxopentanoate synthesis
- Product Name:Methyl 4-Methyl-5-oxopentanoate
- CAS Number:40630-06-6
- Molecular formula:C7H12O3
- Molecular Weight:144.17
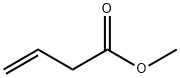
3724-55-8
90 suppliers
$106.00/1g
201230-82-2
1 suppliers
inquiry
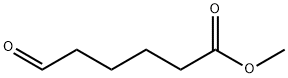
6654-36-0
83 suppliers
$20.00/250mg

40630-06-6
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;acetic acid;acetylacetonatodicarbonylrhodium(l);N-(6-diphenylphosphanylpyridin-2-ylcarbonyl)guanidine in tetrahydrofuran at 40; under 7500.75 Torr; for 4 h;Product distribution / selectivity;Autoclave;
Steps:
V.4.4.2
4. Hydroformylation of Methyl but-3-enoate (12) (Not According to the Invention)Reaction conditions: reactor: autoclave (A); molar ratio of [Rh(CO)2acac]:(1):(12):CH3COOH:standard=1:10:200:(as shown in Table 2): 100; solvent: THF (2 ml); starting concentration of the substrate (12): c0(12)=0.2 M; synthesis gas: CO/H2 (1:1); reaction pressure: 10 bar; reaction temperature: 40° C.; reaction time: 4 h.Possible Products of the Hydroformylation: The turnover frequency (TOF; mol(aldehyde)/mol(catalyst) h-1) was determined from the consumption of synthesis gas. The conversion (in %) and the regioselectivity of the reaction (molar ratio of (13)/(14)) was determined by integration of the characteristic signals of the reaction products formed in the 1H-NMR spectrum of the resulting reaction mixture diluted with CDCl3. Each experiment was repeated at least twice. By-products of this reaction were observed in an amount of <5% in all experiments.
References:
BASF SE US2010/240896, 2010, A1 Location in patent:Page/Page column 10-11
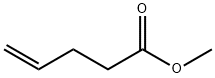
818-57-5
45 suppliers
$98.00/1g-f
201230-82-2
1 suppliers
inquiry

6654-36-0
83 suppliers
$20.00/250mg

40630-06-6
8 suppliers
inquiry
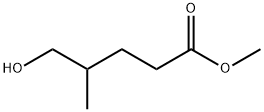
167707-57-5
2 suppliers
inquiry

40630-06-6
8 suppliers
inquiry

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)