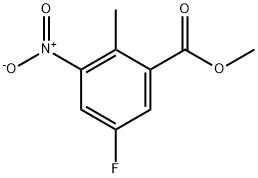
Methyl 5-fluoro-2-methyl-3-nitrobenzoate synthesis
- Product Name:Methyl 5-fluoro-2-methyl-3-nitrobenzoate
- CAS Number:697739-03-0
- Molecular formula:C9H8FNO4
- Molecular Weight:213.16

67-56-1
771 suppliers
$9.00/25ml

850462-64-5
98 suppliers
$11.00/250mg

697739-03-0
166 suppliers
$8.00/250mg
Yield:697739-03-0 96%
Reaction Conditions:
with thionyl chloride for 3 h;Cooling with ice;Reflux;
Steps:
2.212.1 Step 1
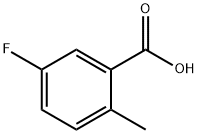
Dissolve 5-fluoro-2-methyl-3-nitrobenzoic acid in 20ml of methanol, add dichlorosulfoxide (728ul, 10.04mmol) under ice bath, warm to reflux for 3h, after the reaction is complete, Spin dry, dilute with ethyl acetate, wash with saturated sodium bicarbonate and saturated sodium chloride respectively, and dry to obtain the target product 1.025g, yield 96%.
References:
CN110963994,2020,A Location in patent:Paragraph 0475; 1148-1150

850462-64-5
98 suppliers
$11.00/250mg

74-88-4
351 suppliers
$15.00/10g

697739-03-0
166 suppliers
$8.00/250mg

850462-64-5
98 suppliers
$11.00/250mg

697739-03-0
166 suppliers
$8.00/250mg

67-56-1
771 suppliers
$9.00/25ml
33184-16-6
327 suppliers
$6.00/1g

697739-03-0
166 suppliers
$8.00/250mg

175278-29-2
122 suppliers
$9.00/1g

697739-03-0
166 suppliers
$8.00/250mg