
methyl 5-methoxy-1-methyl-1H-indole-3-carboxylate synthesis
- Product Name:methyl 5-methoxy-1-methyl-1H-indole-3-carboxylate
- CAS Number:172595-71-0
- Molecular formula:C12H13NO3
- Molecular Weight:219.24

10242-01-0
167 suppliers
$12.00/250mg

74-88-4
341 suppliers
$15.00/10g

172595-71-0
2 suppliers
inquiry
Yield:172595-71-0 94.2%
Reaction Conditions:
Stage #1: 5-Methoxyindole-3-carboxylic acidwith sodium hydride in N,N-dimethyl-formamide at 0 - 5; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 0 - 20; for 2 h;
Steps:
3.1.2. General procedure for the preparation of 2b-h
General procedure: 60% NaH (0.91 g, 22.8 mmol) was added in portions to a solution of 1b-h (11.2 mmol) in dry DMF (35 mL) at 0-5 °C. After addition, the mixture was stirred for 30 min. Iodomethane (33.6 mmol) was then added at 0-5 °C and stirred for 2 h at room temperature. The mixture was then poured into H2O (120 mL) and the resulting solution was extracted with ethyl acetate (50 mL × 3). The organic phase was combined and washed with brine (150 mL × 3), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The obtained residue was purified by flash column chromatography on silica gel to afford 2b-h.
References:
Hu, Yuanyuan;Ruan, Wenchen;Gao, Anhui;Zhou, Yubo;Gao, Lixin;Xu, Meng;Gao, Jianrong;Ye, Qing;Li, Jia;Pang, Tao [Pharmazie,2017,vol. 72,# 12,p. 707 - 713]
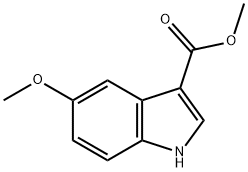
172595-68-5
80 suppliers
$18.00/250mg

74-88-4
341 suppliers
$15.00/10g

172595-71-0
2 suppliers
inquiry
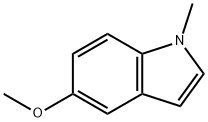
2521-13-3
37 suppliers
$34.00/100mg

172595-71-0
2 suppliers
inquiry
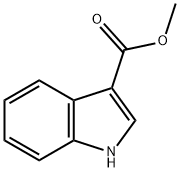
942-24-5
260 suppliers
$6.00/5g

172595-71-0
2 suppliers
inquiry
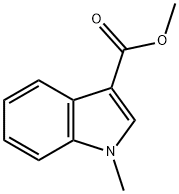
108438-43-3
60 suppliers
inquiry

172595-71-0
2 suppliers
inquiry