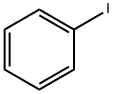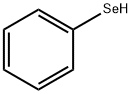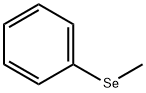
METHYLPHENYLSELENIDE synthesis
- Product Name:METHYLPHENYLSELENIDE
- CAS Number:4346-64-9
- Molecular formula:C7H8Se
- Molecular Weight:171.1
Yield:4346-64-9 100 %Chromat.
Reaction Conditions:
Stage #1: iodobenzenewith selenobenzamide;potassium tert-butylate in dimethyl sulfoxide; for 3 h;Inert atmosphere;Irradiation;
Stage #2: methyl iodide in dimethyl sulfoxide;Inert atmosphere;
Steps:
Representative experimental procedure:
The photochemical reaction was carried out in a three-necked, 10 mL Schlenk tube equipped with a nitrogen gas inlet and a magnetic stirrer. The flask was dried under vacuum, filled with nitrogen and then charged with 10 mL of dried DMSO. 3.75 mmol of tert-BuOK for selenobenzamide (3.25 mmol for anion selenourea and 2.5 mmol for KSeCN) was added to the degassed DMSO under nitrogen, then 2.5 mmol of the nucleophile 1-3 and 0.5 mmol of ArX were added to the reaction mixture. After 3 h of irradiation with a high-pressure Hg lamp, the reaction was quenched by the addition of MeI (6 equiv) in excess and 30 mL of water, and the mixture was then extracted with dichloromethane (3 × 20 mL). The organic extract was washed twice with water, dried and the products were quantified by GC with diphenyl diselenide as internal standard.
References:
Bouchet, Lydia M.;Pe?é?ory, Alicia B.;Argüello, Juan E. [Tetrahedron Letters,2011,vol. 52,# 9,p. 969 - 972] Location in patent:experimental part
1666-13-3
221 suppliers
$10.00/1g
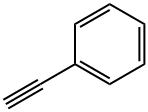
536-74-3
295 suppliers
$10.00/1g

74-88-4
341 suppliers
$15.00/10g
![Benzene, [(2-phenylethynyl)seleno]-](/CAS/20210305/GIF/30665-96-4.gif)
30665-96-4
0 suppliers
inquiry

4346-64-9
2 suppliers
inquiry
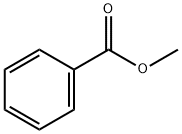
93-58-3
470 suppliers
$14.00/25mL

1666-13-3
221 suppliers
$10.00/1g

4346-64-9
2 suppliers
inquiry
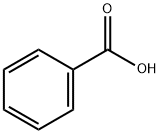
65-85-0
1331 suppliers
$10.00/25g