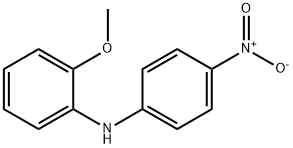
N-(2-METHOXY-PHENYL)-BENZENE-1,4-DIAMINE synthesis
- Product Name:N-(2-METHOXY-PHENYL)-BENZENE-1,4-DIAMINE
- CAS Number:5840-11-9
- Molecular formula:C13H14N2O
- Molecular Weight:214.26
Yield:5840-11-9 59%
Reaction Conditions:
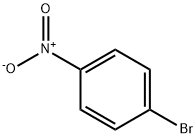
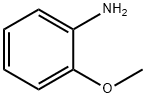
Stage #1: 2-methoxy-phenylamine;para-nitrophenyl bromidewith tris-(dibenzylideneacetone)dipalladium(0);potassium carbonate;XPhos in isopropyl alcohol at 110; for 24 h;Inert atmosphere;Buchwald-Hartwig Coupling;
Stage #2: with potassium tert-butylate;bis(pinacol)diborane in isopropyl alcohol at 110; for 4 h;Inert atmosphere;chemoselective reaction;
Steps:
A 4.2. General procedure A
General procedure: In a dried schlenk flask (25 mL in volume) equipped with a stirring bar were placed with 1-bromo-4-nitrobenzene (0.275 mmol, 1.1 eq.), Pd2(dba)3 (2.3mg, 0.0025 mmol, 1 mol%), XPhos (5.9 mg, 0.0125 mmol, 5 mol%), K2CO3 (69.1 mg, 0.5 mmol, 2.0 eq.) and arylamines (0.25 mmol, 1.0 eq., if solid). After evacuation and refill with dry nitrogen for three times, arylamines (0.25 mmol, 1.0 eq., if liquid) and iPrOH (1.0 mL) were added with syringes under a stream of nitrogen. The resulting mixture was allowed to stir at 110°C for 24 h. Then, B2pin2 (1.0 mmol, 4.0 eq.) and KOt-Bu (0.5 mmol, 2.0 eq.) were added and the mixture was then stirred in the preheated oil bath at 110°C for 4 h. After cooling to room temperature, the crude production was diluted with ethyl acetate and then washed with saturated NaCl solution. The organic layers dried over anhydrous Na2SO4, concentrated in vacuo, and purified by flash column chromatograph to give the pure products.
References:
Liang, Xueting;Xu, Liang;Li, Cuihua;Jia, Xin;Wei, Yu [Tetrahedron,2019,vol. 75,# 6,p. 721 - 731]
103966-66-1
130 suppliers
$7.00/100mg

62-53-3
688 suppliers
$10.00/1g

5840-11-9
11 suppliers
inquiry
729-04-4
0 suppliers
inquiry

5840-11-9
11 suppliers
inquiry
90-04-0
377 suppliers
$5.00/5G

5840-11-9
11 suppliers
inquiry
350-46-9
522 suppliers
$10.60/1gm:

5840-11-9
11 suppliers
inquiry