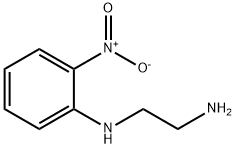
N-(2-NITRO-PHENYL)-ETHANE-1,2-DIAMINE synthesis
- Product Name:N-(2-NITRO-PHENYL)-ETHANE-1,2-DIAMINE
- CAS Number:51138-16-0
- Molecular formula:C8H11N3O2
- Molecular Weight:181.19
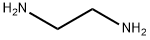
107-15-3
0 suppliers
$11.19/25ML

1493-27-2
513 suppliers
$5.00/10g

51138-16-0
21 suppliers
$45.00/100mg
Yield:51138-16-0 78%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20 - 70; for 21 h;
Steps:
11.A (3-{[2-(2-Nitro-phenylamino)-ethylamino]-methyl}-phenyl)-piperidin-1-yl-methanone.
A. N'-(2-Nitro-phenyl)-ethane-1,2-diamine. To a mixture of ethylenediamine (1.05 g, 17.5 mmol) and K2CO3 (2.44 g, 17.7 mmol) in anhydrous CH3CN (300 mL) heated to 70° C., a solution of o-fluoronitrobenzene (1.25 g, 8.87 mmol) in CH3CN (50 mL) was added dropwise over 2 h. The resulting suspension was stirred at 70° C. for 1 h, and then allowed to cool to 25° C. and stirred for 18 h. The suspension was filtered, and the filtrate was concentrated under reduced pressure. The crude residue was partitioned between H2O (200 mL) and CH2Cl2 (200 mL), and the organic layer was washed with H2O (2×200 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure to provide a bright yellow semisolid (1.5 g, 78%). MS (ESI): mass calculated for C8H11N3O2, 181.09; m/z found, 182.1 [M+H]+. 1H NMR (400 MHz, CDCl3): 8.26 (brs, 1H), 8.17 (dd, J=8.6, 1.6 Hz, 1H), 7.46-7.41 (m, 1H), 6.86 (dd, J=8.7, 0.82 Hz, 1H), 6.63-6.62 (m, 1H), 3.39 (q, J=5.7 Hz, 2H), 3.06 (t, J=5.7 Hz, 2H). 13C NMR (100 MHz, CDCl3): 40.8, 45.7, 113.4, 115.3, 127.1, 136.2, 145.6.
References:
US2005/49239,2005,A1 Location in patent:Page/Page column 16-17
88-73-3
49 suppliers
$14.00/25g
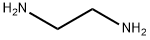
107-15-3
0 suppliers
$11.19/25ML

51138-16-0
21 suppliers
$45.00/100mg

1493-27-2
513 suppliers
$5.00/10g

51138-16-0
21 suppliers
$45.00/100mg
![Carbamic acid, [2-[(2-nitrophenyl)amino]ethyl]-, 1,1-dimethylethyl ester](/CAS/20180601/GIF/834881-63-9.gif)
834881-63-9
3 suppliers
inquiry

51138-16-0
21 suppliers
$45.00/100mg

88-73-3
49 suppliers
$14.00/25g
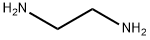
107-15-3
0 suppliers
$11.19/25ML

51138-16-0
21 suppliers
$45.00/100mg

5431-37-8
3 suppliers
$28.60/250mg