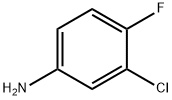
N-(3-CHLORO-4-FLUORO-PHENYL)-2-CYANO-ACETAMIDE synthesis
- Product Name:N-(3-CHLORO-4-FLUORO-PHENYL)-2-CYANO-ACETAMIDE
- CAS Number:219529-31-4
- Molecular formula:C9H6ClFN2O
- Molecular Weight:212.61
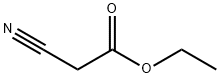
367-21-5
506 suppliers
$5.00/25g

372-09-8
350 suppliers
$24.73/10gm:

219529-31-4
23 suppliers
inquiry
Yield:219529-31-4 94%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in tetrahydrofuran at 20 - 45; for 1.5 h;Product distribution / selectivity;
Steps:
2 (3-Chloro-4-fluoro)-2-cyanoacetamide
EXAMPLE 2 (3-Chloro-4-fluoro)-2-cyanoacetamide A 500-mL round bottomed flask under N2 equipped with overhead stirrer, a condenser, thermocouple and 100 mL dropping funnel was charged with 1 -[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (21.17 g, 0.11 ml) and THF (140 mL). A solution of cyanoacetic acid (10.03 g, 0.118 mol) and 3-chloro-4-fluoroaniline (15.44 g, 0.106 mol) in THF (60 mL) was added to the dropping funnel and added to the reaction flask at 25-45° C. At the end of the addition the reaction became a clear solution. The solution was stirred at ambient temperature for 1.5 hours then poured slowly into water (500 mL) forming a white suspension. The suspension was stirred at room temperature overnight then filtered and the filter cake washed with water and dried under vacuum (50 mm Hg) at 45° C. to provide (3-chloro-4-fluoro)-2-cyanoacetamide as a white solid (21.27 g, 94% yield, 97.5% by HPLC). 1H NMR (300 MHz, DMSO-d6): 10.4 (s, 1H), 7.80 (d, J=8 Hz, 1H), 7.5-7.3 (m, 2H), 3.92 (s, 2H).
References:
US2005/43537,2005,A1 Location in patent:Page/Page column 6

367-21-5
506 suppliers
$5.00/25g

372-09-8
350 suppliers
$24.73/10gm:

693-13-0
525 suppliers
$13.00/1ml
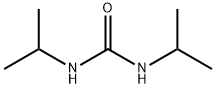
4128-37-4
74 suppliers
$50.00/5mg

219529-31-4
23 suppliers
inquiry