
N-(4-TERT-BUTYLBENZYL)-N-METHYLAMINE synthesis
- Product Name:N-(4-TERT-BUTYLBENZYL)-N-METHYLAMINE
- CAS Number:65542-26-9
- Molecular formula:C12H19N
- Molecular Weight:177.29
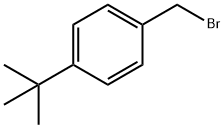
18880-00-7
206 suppliers
$9.00/5g

65542-26-9
22 suppliers
inquiry
Yield:65542-26-9 53.7%
Reaction Conditions:
with sodium hydroxide;sodium carbonate;methylamine in methanol;water;
Steps:
R.19 Production of N-(4-tert-Butylbenzyl)methylamine (Alternative Method)
The thus-obtained product was analyzed by 1H-NMR, and was found to be a mixture of the target compound, starting compounds, and a dibromo compound (10:1:1). Sodium carbonate (10.6 g; 0.10 mol) was added to 40% solution of methylamine in methanol (200 ml). While the mixture was cooled in an ice bath, p-tert-butyl benzyl bromide (22.7 g; 0.10 mol) in methanol (20 ml) was added dropwise thereto. The mixture was removed from the ice bath, and stirred for 41 hours at room temperature. Methanol was removed under reduced pressure, and the residue was taken up in water, followed by extraction with ether (400 ml). The ether layer was extracted with 1N hydrochloric acid twice (200 ml and 100 ml), and the aqueous layer was extracted with ethyl acetate. The aqueous layer was alkalinized with aqueous 2N sodium hydroxide solution, and extracted with ether (400 ml), followed by drying over magnesium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (chloroform:methanol=200:1→100:1→20:1), to thereby yield 9.51 g of the target compound (yield: 53.7%).
References:
US6586633,2003,B1
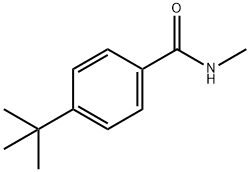
60028-84-4
7 suppliers
inquiry

65542-26-9
22 suppliers
inquiry