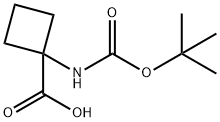
N-Boc-1-aminocyclobutanecarboxylic acid synthesis
- Product Name:N-Boc-1-aminocyclobutanecarboxylic acid
- CAS Number:120728-10-1
- Molecular formula:C10H17NO4
- Molecular Weight:215.25

24424-99-5
856 suppliers
$13.50/25G
22264-50-2
293 suppliers
$11.00/1g

120728-10-1
325 suppliers
$6.00/1g
Yield:120728-10-1 75%
Reaction Conditions:
with sodium hydrogencarbonate in 1,4-dioxane;water at 0 - 20; for 12 h;
Steps:
4.1 Step 1: Synthesis of 1-((tert-butoxycarbonyl)amino)cyclobutane-1-carboxylic acid
To a stirred solution of 1-aminocyclobutane-1-carboxylic acid (2 g, 17 mmol) in 1,4- dioxane: H2O (20: 20 mL), were added NaHCO3 (4.4 g, 57 mmol) and (Boc)2O (4.5 g, 20.4 mmol) at 0°C and stirred the reaction mixture for about 12 hours at room temperature. After complete conversion of starting material, the reaction mixture was washed with EtOAc (30 mL) to remove the impurities, then aqueous layer was acidified with 1N HCl (PH =2-3) and extracted with CH2Cl2 (2x40 mL). The combined organic extracts were washed with water, brine, dried over Na2S04, filtered and evaporated under reduced pressure. The crude residue was purified by silica gel column chromatography by using 35 % EtOAc: n-Hexane as an eluent to afford the desired product (2.8 g, yield: 75%) as an off white solid. 1H MR (300 MHz, CD3OD): δ 2.62-2.53 (m, 2H), 2.25-2.15 (m, 2H), 2.05-1.97 (m, 2H), 1.42 (s, 9H); ES Mass: 238.04 [M+Na]+.
References:
HETERO RESEARCH FOUNDATION;BANDI, Parthasaradhi Reddy;KURA, Rathnakar Reddy;GAZULA LEVI, David Krupadanam;ADULLA, Panduranga Reddy;NEELA, Sudhakar;MOGILI, Narsingam WO2017/21922, 2017, A1 Location in patent:Page/Page column 26
![Cyclobutanecarboxylic acid, 1-[[(1,1-diMethylethoxy)carbonyl]aMino]-, ethyl ester](/CAS/GIF/163554-54-9.gif)
163554-54-9
14 suppliers
inquiry

120728-10-1
325 suppliers
$6.00/1g
![5,7-Diaza-spiro[3.4]octane-6,8-dione](/CAS/GIF/89691-88-3.gif)
89691-88-3
32 suppliers
$60.00/10mg

120728-10-1
325 suppliers
$6.00/1g

35120-18-4
151 suppliers
$6.00/100mg

120728-10-1
325 suppliers
$6.00/1g

24424-99-5
856 suppliers
$13.50/25G

120728-10-1
325 suppliers
$6.00/1g