
N-Ethylmethylamine synthesis
- Product Name:N-Ethylmethylamine
- CAS Number:624-78-2
- Molecular formula:C3H9N
- Molecular Weight:59.11
Yield:624-78-2 90%
Reaction Conditions:
with hydrogen;sodium hydroxide;Raney nickel at 65 - 67; under 22502.3 Torr; for 4.3 h;Product distribution / selectivity;Autoclave;Industry scale;
Steps:
1
Example 1According to the InventionIntroduce successively into a 250 lhydrogenation autoclave equipped with a stirring system and a heating/cooling system are 89.5 kg of monomethylamine (commercial 40.5 wt % aqueous solution, i.e. 36.2 kg of anhydrous MMA), around 5.5 kg of Raney nickel (weight concentration of nickel in the catalyst greater than 82%) and around 0.65 kg of sodium hydroxide that is introduced in the form of an aqueous solution with a titre of 450 g/l.The whole assembly is placed under hydrogen pressure and heated at a temperature of around 65-67° C. Acetaldehyde (56.3 kg) is then introduced into the autoclave over a period of around 3.3 hours. The hydrogen pressure is maintained at around 3 MPa, over the duration of the reaction. After adding all of the acetaldehyde, the reaction is maintained at temperature and under hydrogen pressure until the consumption of hydrogen stops (i.e. around 1 hour).At the end of the reaction, the N-ethylmethyl-amine selectivity, with respect to the acetaldehyde, is 85.6 mol %, and the molar yield of EMA is 93% relative to the starting monomethylamine.The stirring is stopped, the autoclave is degassed and the catalyst is left to settle. The supernatant liquid is then withdrawn and subjected to a fractional distillation under atmospheric pressure in a column comprising around 15 to 20 theoretical plates. After removing the light fractions, the high-purity EMA is recovered via a side stream at a column height of around 90%, with a distillation yield (degree of recovery of EMA relative to the EMA in the crude reaction medium) of 95%.The composition of organic constituents of the high-purity EMA is determined quantitatively and qualitatively by gas chromatography (with internal calibration). The concentration of residual water in the EMA is assayed by potentiometric titration according to the Karl-Fischer method.Thus, an EMA having a purity of 99.86% by weight is obtained, the composition of which is presented in the table below:
References:
Ruppin, Christophe;Dufour, Jacqueline US2011/166387, 2011, A1 Location in patent:Page/Page column 3
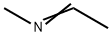
6898-67-5
0 suppliers
inquiry

624-78-2
251 suppliers
$16.00/1g
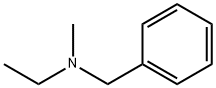
4788-37-8
57 suppliers
$29.30/10g

624-78-2
251 suppliers
$16.00/1g
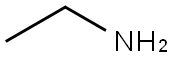
75-04-7
407 suppliers
$10.00/20mg

624-78-2
251 suppliers
$16.00/1g
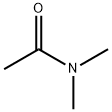
127-19-5
881 suppliers
$5.00/25g
598-56-1
168 suppliers
$22.57/100 mL

624-78-2
251 suppliers
$16.00/1g
616-39-7
129 suppliers
$5.00/1g

