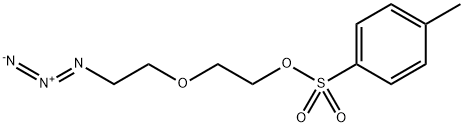
N3-PEG2-Tos synthesis
- Product Name:N3-PEG2-Tos
- CAS Number:182347-24-6
- Molecular formula:C11H15N3O4S
- Molecular Weight:285.32
Yield:182347-24-6 90%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 24 h;
Steps:
1 2-(2-Azidoethoxy) ethyl 4-methylbenzenesulfonate (105)
To a solution of compound 103 (1.4 g, 10.7 mmol, leq), Et3N (3.0 mL, 21.4 mmol, 2.0 eq) in DCM (60 mL) at 0°C was added TsCl (2.4 g, 12.8 mmol, 1.2 eq). The resulting mixture was stirred at r.t. for 24 h and was quenched with cold saturated Na2S2Cb. The water layer was then extracted with DCM (3 x 50 mL). The organic layers were finally combined, washed with water, brine, and dried over anhydrous Na2S04, and concentrated under reduced pressure. The residue was purified by flash chromatography (DCM /Ethyl acetate 3: 1 to 1 :2) to afford product 105 (2.7 g, 90%). H NMR (400 MHz, CDCb) d 7.81 (d, J= 8.3 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 4.18-4.16 (m, 2H), 3.71-3.69 (m, 2H), 3.62-3.59 (m, 2H), 3.32 (t, J= 5.1 Hz, 2H), 2.45 (s, 3H).
References:
WO2020/72955,2020,A1 Location in patent:Page/Page column 143; 202-203
7460-82-4
217 suppliers
$6.00/1g

182347-24-6
20 suppliers
inquiry
118591-58-5
83 suppliers
$82.00/100mg

182347-24-6
20 suppliers
inquiry

118591-58-5
83 suppliers
$82.00/100mg

98-59-9
585 suppliers
$9.00/5g

182347-24-6
20 suppliers
inquiry

98-59-9
585 suppliers
$9.00/5g

182347-24-6
20 suppliers
inquiry
