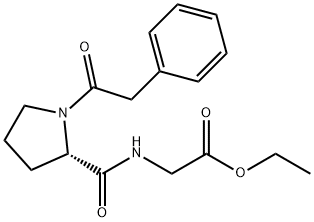
Noopept synthesis
- Product Name:Noopept
- CAS Number:157115-85-0
- Molecular formula:C17H22N2O4
- Molecular Weight:318.37
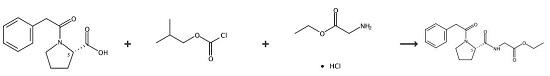
1. Perform the carboxylation of N-phenylacetyl-L-proline and ethyl glycine in the presence of isobutyl chloroformate.
2. Add slowly isobutyl chloroformate (1.17 g, 8.6 mmol) to a solution of N-phenylacetyl-L-proline (2.0 g, 8.6 mmol) dissolved in a mixed solvent of THF and dichloromethane (1:1; THF/dichloromethane (v/v)).3.Stir the mixture for 4 h at 0 ~5 °C
Molecular Mechanism Underlying the Action of Substituted Pro-Gly Dipeptide Noopept
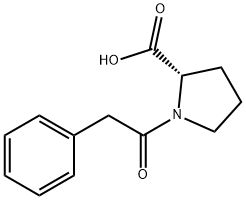
2752-38-7
25 suppliers
$237.00/500mg

157115-85-0
370 suppliers
$5.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: N-methylmorpholine / CHCl3 / 0.03 h / -10 °C
2: N-methylmorpholine / CHCl3; dimethylformamide / 1.5 h / -5 - 20 °C
References:
Gudasheva;Voronina;Ostrovskaya;Rozantsev;Vasilevich;Trofimov;Kravchenko;Skoldinov;Seredenin [European Journal of Medicinal Chemistry,1996,vol. 31,# 2,p. 151 - 157]
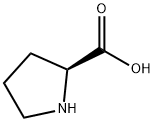
147-85-3
1059 suppliers
$6.00/25g

157115-85-0
370 suppliers
$5.00/100mg