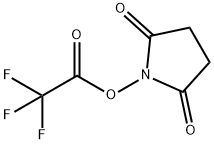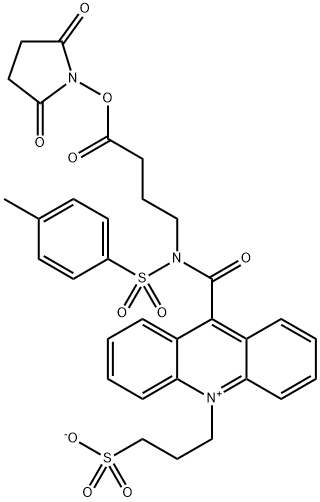
NSP-SA-NHS synthesis
- Product Name:NSP-SA-NHS
- CAS Number:199293-83-9
- Molecular formula:C32H31N3O10S2
- Molecular Weight:681.73
Yield:199293-83-9 92%
Reaction Conditions:
with 1,1'-carbonyldiimidazole in acetonitrile at 20; for 8 h;Inert atmosphere;Solvent;
Steps:
1; 1 Example 1:
See the chemical reaction formula shown in Figure 1,From 10-(3-sulfopropyl)-N-p-toluenesulfonyl-N-(3-carboxypropyl) acridine-9-carboxamide,That is, the compound of formula C prepares 9-[[[4-[(2,5-dioxo-1-pyrrolidinyl)oxy]-4-oxobutyl][(4-methylphenyl)sulfonyl ]Amino]carbonyl]-10-(3-sulfopropyl)-acridine internal salt,Formula D compound: after dissolving 200 mg of compound C with acetonitrile,The reaction solution was not dissolved, and 150 mg of N,N'-carbonyldiimidazole was added.After being ventilated by N2, wrapped in foil paper, activated at room temperature for 2h, the reaction solution gradually dissolved,Add 194 mg of N-hydroxysuccinimide and react at room temperature for 6h.A yellow solid precipitated, HPLC tracking showed that the raw material reaction was complete,Add 6 times the volume of acetonitrile tert-butyl methyl ether and stir to crystallize, suction filter, the filter cake was washed with a small amount of tert-butyl methyl ether, the solid was collected and suction dried213 mg of yellow solid was obtained, HPLC>98%, yield: 92%, stored at -20°C under shading.
References:
CN111170994,2020,A Location in patent:Paragraph 0023-0025

98-59-9
589 suppliers
$9.00/5g

199293-83-9
93 suppliers
inquiry
![Butanoic acid, 4-[[(4-methylphenyl)sulfonyl]amino]-, methyl ester](/CAS/20210305/GIF/118429-43-9.gif)
118429-43-9
0 suppliers
inquiry

199293-83-9
93 suppliers
inquiry
5132-80-9
6 suppliers
inquiry

199293-83-9
93 suppliers
inquiry
![3-[9-(((3-(carboxypropyl)[4-Methxylphenyl]sulfonyl)aMine)carboxyl]-10-acridiniuMyl)-1-propanesulfonate inner salt](/CAS/20180808/GIF/211106-69-3.gif)