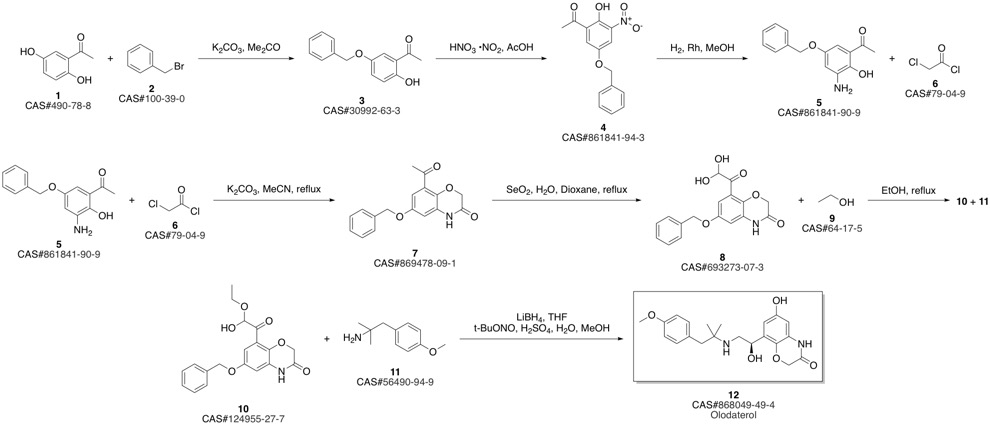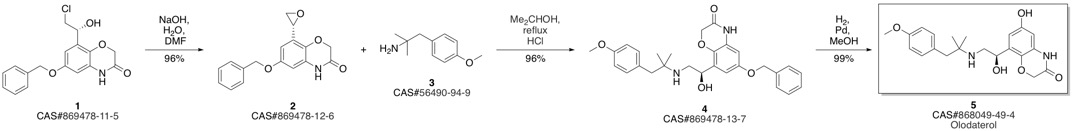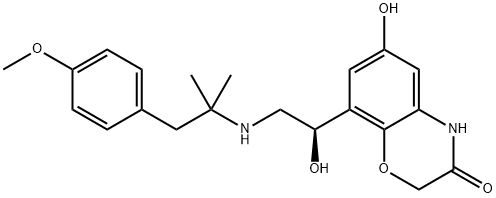
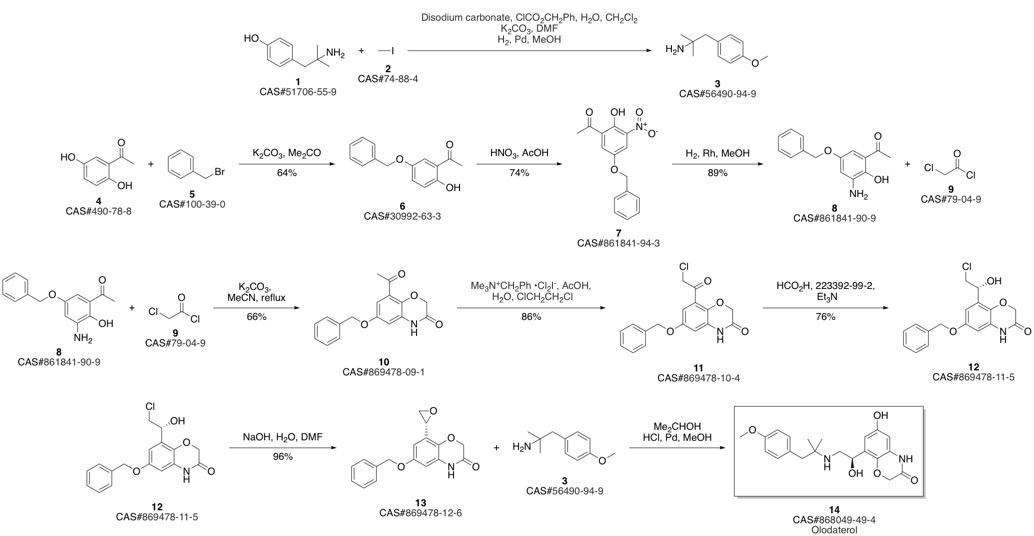
Olodaterol synthesis
- Product Name:Olodaterol
- CAS Number:868049-49-4
- Molecular formula:C21H26N2O5
- Molecular Weight:386.44
Bouyssou, Thierry; Hoenke, Christoph; Rudolf, Klaus; Lustenberger, Philipp; Pestel, Sabine; Sieger, Peter; Lotz, Ralf; Heine, Claudia; Buettner, Frank H.; Schnapp, Andreas; Konetzki, Ingo. Discovery of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24h bronchodilatory efficacy. Bioorganic & Medicinal Chemistry Letters. Volume 20. Issue 4. Pages 1410-1414. Journal. (2010).
![8-[(1R)-1-Hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-6-(phenylmethoxy)-2H-1,4-benzoxazin-3(4H)-one](/CAS/20180703/GIF/869478-13-7.gif)
869478-13-7
47 suppliers
inquiry

868049-49-4
87 suppliers
$56.00/1 mg
Yield:868049-49-4 99.27%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 50; under 2250.23 Torr;
Steps:
1.3 3) Preparation of crude odastatol hydrochloride
60 g (0.117 mol) prepared in the step 2) 6-Benzyloxy-8-{(R)-1-hydroxy-2-[2-(4-methoxy-phenyl)- 1,1-Dimethyl-ethylamino]-ethyl}-4H-benzo[1,4]oxazin-3-one, 600 ml of methanol and 8 g of 10% palladium on carbon were added to the reaction flask and heated to 50 ° C. , hydrogenation under 3 bar of hydrogen pressure. Then, the catalyst was removed by suction filtration, and the solvent was evaporated to dryness to dryness to give 49.1 g of white foamy solid, yield: 99.27%. Purity: 95.3%.
References:
Shanghai Fangyu Health Pharmaceutical Technology Co., Ltd.;Yu Xiong;Zhang Yuanwei;Yuan Xilun CN109096218, 2018, A Location in patent:Paragraph 0074; 0079-0080
![6-benzyloxy-8-((R)-2-chloro-1-hydroxy-ethyl)-4H-benzo[1,4]-oxazin-3-one](/CAS/20180702/GIF/869478-11-5.gif)
869478-11-5
24 suppliers
inquiry

868049-49-4
87 suppliers
$56.00/1 mg