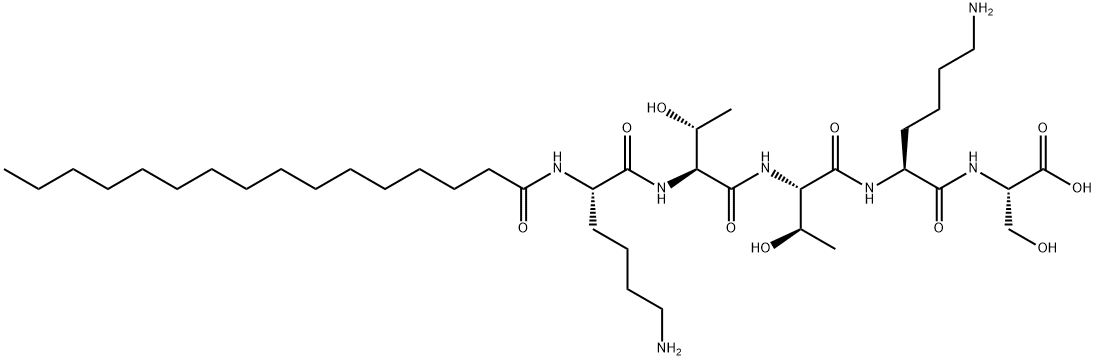
Palmitoyl Pentapeptide synthesis
- Product Name:Palmitoyl Pentapeptide
- CAS Number:214047-00-4
- Molecular formula:C39H75N7O10
- Molecular Weight:802.05
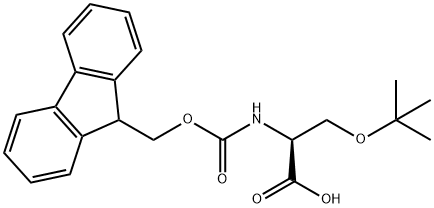
71989-33-8
425 suppliers
$6.00/5g
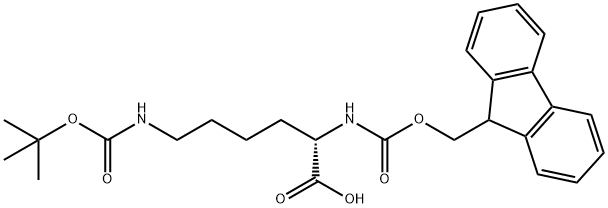
71989-26-9
462 suppliers
$5.00/1g
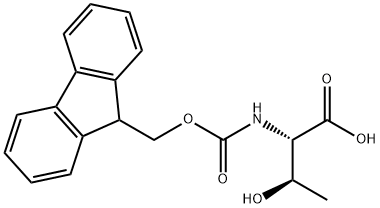
73731-37-0
293 suppliers
$14.30/5g
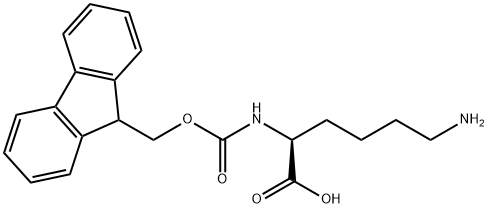
105047-45-8
219 suppliers
$8.00/1g

57-10-3
550 suppliers
$5.00/250mg

214047-00-4
350 suppliers
inquiry
Yield:214047-00-4 24.9%
Reaction Conditions:
Stage #1: Fmoc-Ser(tBu)-OHwith N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide; for 6 h;
Stage #2: with 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethyl-formamide;
Stage #3: Fmoc-Lys(tert-butoxycarbonyl);Fmoc-Thr-OH;Fmoc-Lys-OH;1-hexadecylcarboxylic acidFurther stages;
Steps:
1.1-3 1-3: Palmitoyl-KTTKS peptide synthesis and purification
1) Synthesis of pentapeptide (lysine-threonine-threonine-lysine-serine, KTTKS) In a glass reactor, 74 mg (100 μmol equivalent) of 2-Chlorotrityl chloride resin (Nova Biochem, Inc) was swelled in DMF as a solvent for 2 hours, then the solvent was removed, and Fmoc-Ser(tBu)-OH was 95.9. Mg (250 μmol equivalent) was activated with N,N-Diisopropylethylamine (DIEA) and added.After 4 hours, the solvent was removed and treated twice with 2% DBU to remove Fmoc.The resin from which Fmoc was removed was treated with DMF three times, and reacted with a solution of Fmoc-Lys(Boc)-OH activated with HOBt and DIC at room temperature.In this process, the NH2-protected peptide (lysine-threonine-threonine-lysine-serine) resinobtained by continuously reacting threonine, threonine, and lysinewas washed three times with DMF 2) Palmitoyl-pentapeptide (lysine-threonine-threonine-lysine-serine, KTTKS) After washing theNH2-protected peptide (lysine-threonine-threonine-lysine-serine)-resinsynthesized in 1)with DMF, 10 equivalents of Palmitic acid and 10 equivalents of HOBt (10 equivalents), DIC (10 Equivalent) was mixed, and a coupling reaction was carried out overnight at room temperature.After the reaction was completed, it was washed 3 times with DMF and 4 times with MC, and then dried.Dried palmitoyl-pentapeptide (lysine-threonine-threonine-lysine-serine) resin was mixed with trifluoroacetic acid: water: TIS (95: 2.5: 2.5 (v/v)) at room temperature for 2 to 3 hours During the reaction, the Boc group, which is the protecting group of the functional group present in the side chain of the amino acid residue constituting the peptide, was removed, palmitoyl-pentapeptide (lysine-threonine-threonine-lysine-serine) was separated from the resin, and then cold diethyl ether The peptide was precipitated and dried. 3) High purity purification After drying, crude palmitoyl-pentapeptide (lysine-threonine-threonine-lysine-serine) was purified by reverse phase high-performance liquid chromatography using water containing 0.1% trifluoroacetic acid and acetonitrile as a solvent.The obtained peptide was purified using HPLC and then lyophilized to obtain 20 mg of palmitoyl-pentapeptide (Palmitoyl-KTTKS) having a purity of 95% or higher (yield 24.9%, purity 99.07%, molecular weight [M+]). H]+802.6).
References:
KR2020/127075,2020,A Location in patent:Paragraph 0044; 0066-0074