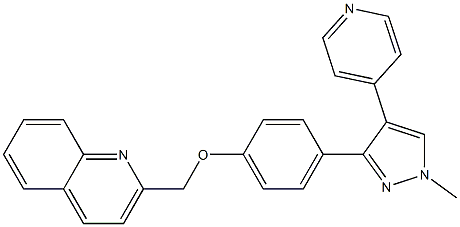
PF-2545920 synthesis
- Product Name:PF-2545920
- CAS Number:1292799-56-4
- Molecular formula:C25H20N4O
- Molecular Weight:392.4525

871507-11-8
3 suppliers
inquiry
54245-42-0
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1292799-56-4
73 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 85; for 0.0833333 h;
Steps:
3.3.2. Radiosynthesis of [11C]MP-10
A stream of [11C]CH3I in helium was bubbled for 5 min into a solution of 6 (1.5-2.0 mg) in DMF (0.18 mL) and 30 μL of sodium hydride in DMF solution (3 mg of NaH (95%) in 250 μL DMF) at 0 °C (ice-water bath). The sealed reaction vessel was heated at 85 °C (oil bath) for 5 min. The reaction was quenched by adding HPLC solvent (1.8 mL), and the diluted solution was injected to a high-performance liquid chromatography (HPLC) Agilent Zorbax SB-C18 reverse phase column (9.4 × 250 mm); the product was eluted by using acetonitrile/0.1 M ammonium formate buffer (40:60, v/v) at pH 4.5 as the mobile phase, at a flow rate of 4.0 mL/min and the UV detection was at 254 nM. Under these conditions, the retention time of the precursor 6 was 12.1 min; the retention time of the [11C]MP-10 was 19.90 min; the retention time of the other isomer [11C]8 was 22.53 min. The [11C]MP-10 product was collected into a vial containing 50 mL milli-Q water and passed through a Sep-Pak Plus C-18 cartridge (Waters, Milford, MA, USA), in which the product was trapped. The trapped product was eluted with ethanol (0.6 mL) followed by 5.4 mL of 0.9% saline. After sterile filtration into a dose vial, the final product was ready for quality control (QC) analysis and animal studies. The HPLC was performed on Agilent SB C-18 reverse phase analytic HPLC column (250 mm × 4.6 mm, 5 μA) and UV detection at 254 nm wavelength. The mobile phase was acetonitrile/0.1 M ammonium formate buffer (45:55, v/v) using 1.2 mL/min flow rate. Under these conditions the retention time of [11C]MP-10 was 10.4 min. The radioactive dose sample was authenticated by co-injection with the cold reference compound MP-10. The radiochemical purity was >92%, the chemical purity was >95%, the labeling yield was 45% (n = 15, decay corrected to EOB) and the specific activity was >370 GBq/μmol (decay corrected to EOB).
References:
Tu, Zhude;Fan, Jinda;Li, Shihong;Jones, Lynne A.;Cui, Jinquan;Padakanti, Prashanth K.;Xu, Jinbin;Zeng, Dexing;Shoghi, Kooresh I.;Perlmutter, Joel S.;MacH, Robert H. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 5,p. 1666 - 1673] Location in patent:experimental part