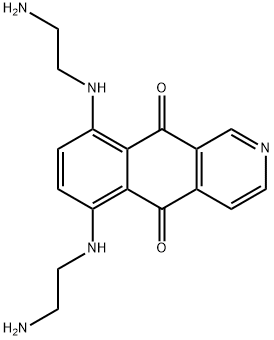
Pixantrone synthesis
- Product Name:Pixantrone
- CAS Number:144510-96-3
- Molecular formula:C17H19N5O2
- Molecular Weight:325.37
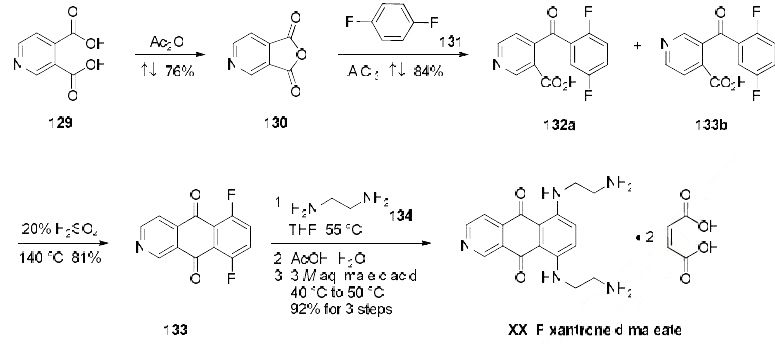
The manufacturing scale synthesis of pixantrone dimaleate relies on several process modifications, from the original synthesis reported by Krapcho in 1994.169 This modified procedure has provided active pharmaceutical ingredient (API) in high purity (>99%) and is acceptable for use in pharmaceutical applications (the scheme). Beginning with pyridine 3,4-dicarboxylic acid (129), generation of the corresponding anhydride 130 proceeded in 76% yield upon treatment with refluxing Ac2O. Next, an AlCl3-promoted Friedel-Crafts reaction of 1,4-difluorobenzene (131) with 130 under reflux conditions provided a mixture of nicotinic acid isomers 132a/132b in 84% yield, which were carried directly to the next step. Cyclization with fuming H2SO4 yielded the desired difluorobenzoisoquinoline- dione core 133, which was further functionalized with ethylenediamine (134) to provide the free base of pixantrone. Subjection of the pixantrone free base to aqueous acetic anhydride and maleic acid provided pixantrone dimaleate (XX) in 92% yield over 3 steps.
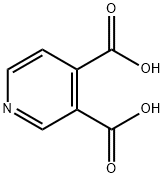
490-11-9
333 suppliers
$6.00/5g

144510-96-3
97 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 3 steps
1: 76 percent / acetic anhydride / 2 h / Heating
2: 1.) AlCl3, 2.) f.H2SO4 / 1.) reflux, 22 h, 2.) from 135 to 140 deg C, 3 h
3: pyridine / 1.) RT, 12 h, 2.) 50 deg C, 2 h
References:
Krapcho, A. Paul;Petry, Mary E.;Getahun, Zelleka;Landi, John J.;Stallman, John;et al. [Journal of Medicinal Chemistry,1994,vol. 37,# 6,p. 828 - 837]
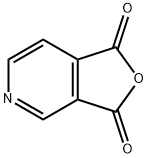
4664-08-8
138 suppliers
$6.00/250mg

144510-96-3
97 suppliers
inquiry
![Benz[g]isoquinoline-5,10-dione, 6,9-dihydroxy-](/CAS/20200331/GIF/4589-37-1.gif)
4589-37-1
0 suppliers
inquiry

144510-96-3
97 suppliers
inquiry
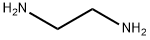
107-15-3
9 suppliers
$11.19/25ML
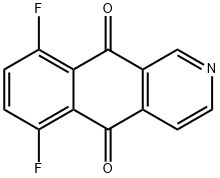
144511-13-7
65 suppliers
$30.00/250mg

144510-96-3
97 suppliers
inquiry