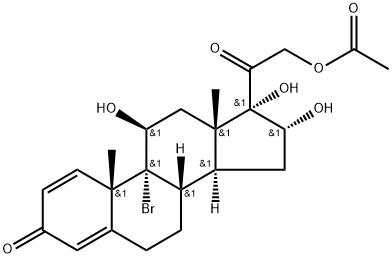
Prednisone Impurity 13 synthesis
- Product Name:Prednisone Impurity 13
- CAS Number:91160-89-3
- Molecular formula:C23H29BrO7
- Molecular Weight:497.38
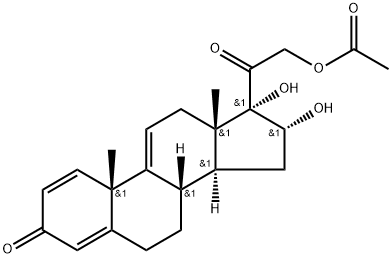
77017-20-0
39 suppliers
$640.00/10mg

91160-89-3
14 suppliers
inquiry
Yield:91160-89-3 94.5%
Reaction Conditions:
with N-Bromosuccinimide;perchloric acid in tetrahydrofuran;water at 20; for 1 h;Large scale;
Steps:
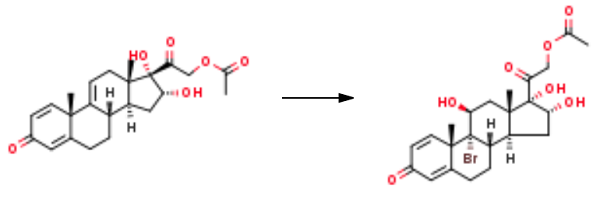
2 Example 2: 9α-bromo-11β,16α,17α,21-tetrahydroxypregnane-1,4-diene-3,20-dione-21-acetate (intermediate 4)
Water (6L), NBS (0.62kg, 3.48mol)And 1.75 mol/L perchloric acid (3.3 L, 5.78 mol) was added to Intermediate 3 (1 kg, 2.50 mol)The solution was stirred at room temperature for 1 h in THF (26 L).The reaction solution was neutralized with a saturated sodium hydrogen carbonate solution (3 L), and added to purified water (100 L).Stirring at room temperature, suction filtration,The filter cake was washed with water to give Intermediate 4 (1.17 kg, 94.5%).
References:
CN109384827,2019,A Location in patent:Paragraph 0039; 0042-0043

37413-91-5
184 suppliers
$50.00/100mg

91160-89-3
14 suppliers
inquiry

37413-91-5
184 suppliers
$50.00/100mg

91160-89-3
14 suppliers
inquiry