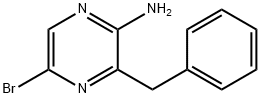
Pyrazinamine, 5-phenyl-3-(phenylmethyl)- synthesis
- Product Name:Pyrazinamine, 5-phenyl-3-(phenylmethyl)-
- CAS Number:70217-86-6
- Molecular formula:C17H15N3
- Molecular Weight:261.32
Yield:70217-86-6 92%
Reaction Conditions:
with [1,4-bis(diphenylphosphino)butane] palladium(ll) dichloride;cis-dichlorobis(benzonitrile)palladium(II);sodium carbonate in ethanol;water;toluene at 105; for 5 h;Inert atmosphere;
Steps:
Synthesisof 3-benzyl-5-phenylpyrazine-2-amine (6).
3-Benzyl-5-bromopyrazine-2-amine (5) (1.32g, 5 mmol, 1 eq.) and phenylboronic acid (915 mg, 7.5 mmol, 1.5 eq.) were dissolved in toluene (70 mL) and stirred at room temperature. Ethanol (20 ml) and 1 M Na2CO3 aq. (48 mL) were added to the reaction mixture. After vacuum deaeration and argon gas protection, 1,4-bis(diphenylphosphino)butane-palladium(II)chloride (190 mg, 0.31 mmol, 14% of compound 5) and bis(benzonitrile)palladium(II)chloride (120 mg, 0.31 mmol, 9% of compound 5) in 30 mL of toluene was added into the solution and the mixture was deaerated again and stirred for 5 hours at 105 °C under argon atmosphere. After cooling to room temperature, the solution was filtered through a diatomite pad to remove the palladium catalyst. The solution was extracted with ethyl acetate, and the brown organic phase was washed with water and brine, dried over Na2SO4 and evaporated. The resulting residue was purified by silica gel column chromatography using petroleum ether/ethyl acetate = 2/1 (v/v), affording 3-benzyl-5-phenylpyrazine-2-amine(6) as a yellow solid (1.2 g, 92%). 1H NMR (300 MHz, DMSO-d6): δ 8.43 (s,1H), 7.92-7.90(2H, t, J = 6.9 Hz), 7.43-7.39 (2H,t, J = 7.2 Hz), 7.36-7.25 (5H, m),7.21-7.18 (1H, t, J = 5.0 Hz), 6.42(2H, s), 4.09 (2H, s); ESI-MS calcd. for C17H15N3: 261.1, found: 262.0 (M+H+).
References:
Yuan, Ming-Liang;Jiang, Tian-Yu;Du, Lu-Pei;Li, Min-Yong [Chinese Chemical Letters,2016,vol. 27,# 4,p. 550 - 554] Location in patent:supporting information
196959-78-1
2 suppliers
inquiry

70217-86-6
8 suppliers
inquiry

93554-83-7
24 suppliers
inquiry

70217-86-6
8 suppliers
inquiry
![Methanesulfonic acid, 1,1,1-trifluoro-, 5-[[(4-methylphenyl)sulfonyl]amino]-6-(phenylmethyl)-2-pyrazinyl ester](/CAS/20210305/GIF/671240-61-2.gif)
671240-61-2
0 suppliers
inquiry

70217-86-6
8 suppliers
inquiry
![Benzenesulfonamide, 4-methyl-N-[5-phenyl-3-(phenylmethyl)-2-pyrazinyl]-](/CAS/20210305/GIF/671240-62-3.gif)
671240-62-3
0 suppliers
inquiry

70217-86-6
8 suppliers
inquiry