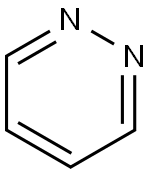
Pyridazine synthesis
- Product Name:Pyridazine
- CAS Number:289-80-5
- Molecular formula:C4H4N2
- Molecular Weight:80.09
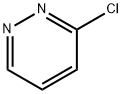
1120-95-2
138 suppliers
$50.00/100 mg

289-80-5
238 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
with potassium acetate;palladium diacetate;bis(pinacol)diborane;tricyclohexylphosphine in 1,4-dioxane at 110; for 0.166667 h;Inert atmosphere;
Steps:
2. General experimental procedures
General procedure: The Miyaura borylation reactions were carried out as follows: Chloropyrazines (3.0 mmol), B2pin2 (838 mg, 3.3 mmol), Pd(OAc)2 (14 mg, 2 mol %), PCy3 (34 mg, 4 mol %) and AcOK (750 mg, 7.5 mmol) was added in a 50 ml three necked flask fitted with a condenser, and dioxane (15 ml) was added at last. Then the reaction mixture was stirred at a preheated oil bath 110 oC under nitrogen atmosphere for 10 min. After complete completion of starting material checked by TLC, the reaction was cooled to room temperature, EtOAc (20 ml) was added. After filtration through Celite and concentration under vacuo, the resulting residue was precipitated from n-hexane:Et2O to afford corresponding boronic esters.
References:
Lu, Hongtao;Wang, Shengqiang;Li, Jingya;Zou, Dapeng;Wu, Yusheng;Wu, Yangjie [Tetrahedron Letters,2017,vol. 58,# 9,p. 839 - 842] Location in patent:supporting information