
(R)-1-N-BOC-2-CYANO-PIPERIDINE synthesis
- Product Name:(R)-1-N-BOC-2-CYANO-PIPERIDINE
- CAS Number:940000-26-0
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27
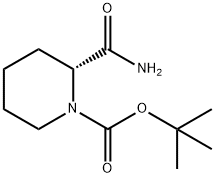
848488-91-5
73 suppliers
$66.00/50 mg

940000-26-0
50 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with triethylamine;trifluoroacetic anhydride in tetrahydrofuran at 0; for 1 h;
Steps:
Z-18.b
b) 1, 1-Dimethylethyl (2R)-2-cyano-1-piporidinecarboxylate. To a cold (0 °C) solutionof 1,1-dimethylethyl (2J?)-2-(aminocarbonyl)-1-piperidinecarboxylate (269 mg, 1.17mmol) in THF (10 mL) was added triethylamme (0,33 mL, 2.34 mmol) and thentrifluoroacetic anhydride (0.17 mL, 1 J 7 mmol). The mixture was stirred at 0 °C for1 h and concentrated in vacuo. The residue was taken up in EtOAc and washedsuccessively with sodium bicarbonate, 0,5 NHCl and brine. The organics were driedover NasSO-j, filtered and concentrated to give 1, 1-dimethylethyl(2/i?)-2-cyano-1-piperidinecarboxylate (255 mg, 99%) as a crystalline solid uponstanding, 1H NMR (CDCl3) δ 5.23 (br, 1 H), 4.05 (br, 1 H), 2.93 (br, 1 H), 1,93- 1.39 (m,6 H), 1.46 (s, 9 H).
References:
SHIONOGI & CO., LTD. WO2006/116764, 2006, A1 Location in patent:Page/Page column 121; 151
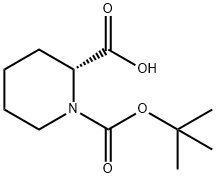
28697-17-8
239 suppliers
$7.00/1g

940000-26-0
50 suppliers
inquiry