
(R)-3,4-DIHYDROXY-BUTYRIC ACID METHYL ESTER synthesis
- Product Name:(R)-3,4-DIHYDROXY-BUTYRIC ACID METHYL ESTER
- CAS Number:88246-12-2
- Molecular formula:C5H10O4
- Molecular Weight:134.13

617-55-0
175 suppliers
$6.00/5g
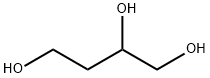
3068-00-6
266 suppliers
$10.00/5g
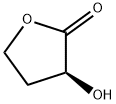
52079-23-9
104 suppliers
$40.00/100mg

497-23-4
228 suppliers
$6.00/1g

88246-12-2
8 suppliers
inquiry
Yield:97.9 - 98.8 % ee
Reaction Conditions:
with hydrogen in Dimethyl ether at 56 - 110; under 120012 Torr; for 0.5 - 2 h;Product distribution / selectivity;
Steps:
1; 2; 3
HYDROGENATION OF DIMETHYL MALATE:; The following conditions were used Hydrogen pressure 0,1, 10 and 30 bar Substrate 0,4 and 1,5 mol% Catalyst CuMn AI203, particle size 90-180 um Samples are taken at regular intervals after the substrate feed is started. Experiment 1: This was performed according to the procedure described above in Materials, Equipment and Activation Procedure. The reaction temperature was 56°C, Hydrogen pressure was 10bar, DME pressure was 150bar, substrate concentration in solution was 0.4 mol%, and 10G of catalyst were used. The following results were obtained: Time on DM L DE BT HGB H-THF HGB equiv Selectivity stream area% area% 5 67.5 0.22 15.16 0.14 15.86 0.1 31.02 95.4% 13 58.4 0.09 21.45 0.27 18.29 0.07 39.74 95.5% 20 63.1 0.15 19.8 0.37 15.6 0.07 35.4 95.9% 30 67.8 0.07 18.2 0.58 12.39 0.06 30.59 95.0% 60 76.5 0.04 15 0.48 7.22 0.04 22.22 94.6% DM = dimethyl malate L = 2 (5H)-Furanone DE = 3,4-dihydroxy methyl butyrate ester BT = butanetriol HGB = 3-hydroxy-gammabutyrolactone (produced by in-situ cyclisation of 3,4-dihydroxy methyl butyrate ester) H-THF = hydroxy-THF Notes: HGB EQUIVALENT = area%"HGB"+ area%"Dihydroxy ester" (as hydroxy ester can be cyclise to HGB) SELECTIVITY DEFINED AS (AREA% USEFUL PRODUCTS) / (AREA % OF ALL PRODUCTS) HGB (S) was obtained in 97.9% ee Experiment 2: This was performed according to the procedure described above in Materials, Equipment and Activation Procedure. The reaction temperature was 110°C, Hydrogen pressure was 10bar, DME pressure was 150bar, substrate concentration in solution was 1.5 mol%, and 5.9g of catalyst were used. The following results were obtained : Time on DM L DE BT HGB H-THF HGB equiv Selectivity stream area% area% 5 64.1 0.55 18.22 1.6 15.3 33.52 93.4% 15 72.6 0.24 15.38 0.9 10 0.1 25.38 92.6% 30 77.6 0.16 13.3 0.5 7.8 0.1 21.1 94.2% Note: HGB (S) obtained in 98.8% ee Conclusion: These experiments illustrate high selectivity for HGB in the hydrogenation of dimethyl malate EXPERIMENT-MEOH removal and the effect on the conversion of LDMM to HGB Experiment 3: This was performed according to the procedure described above in Materials, Equipment and Activation Procedure. The reaction temperature was 80°C, Hydrogen pressure was 10bar, DME pressure was 150bar, substrate concentration in solution was 0.4 mol%, and 87g of catalyst were used. After 2 hours on stream, the product was found to contain 39.8 area% starting material and 51.5 area% HGB-EQUIVALENT (as defined in Example 1). The product was formed with 85.5% selectivity. The collected product from this experiment, after depressurisation and removal of Methanol, was used as the feed for another hydrogenation, under otherwise identical conditions. The following results were obtained. Time on DM area% L DE BT HGB HGB Selectivity stream equiv area% 50 14.7 5.3 15.2 8 47.4 62.6 73.4% 60 18. 8 4.4 14. 8 11.7 45.5 60.3 74.3% 75 21.15 3.7 21.1 11.5 43.2 64. 3 81.5% 90 13. 4 7. 1 5.5 59.1 66.2 76.4% 105 26.7 3.4 17.5 8.7 40.6 58. 1 79.3% 120 25.6 2.3 17.7 9.5 42 59.7 80. 2% Conclusions : When the reaction products from one incomplete hydrogenation are depressurised and Methanol removed by evaporation, then re-hydrogenated the conversion is increased FROM-50% TO-60%, and the selectivity remains approximately constant.
References:
WO2005/23737,2005,A1 Location in patent:Page/Page column 10-12
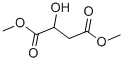
38115-87-6
55 suppliers
inquiry

88246-12-2
8 suppliers
inquiry
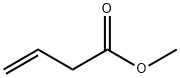
3724-55-8
87 suppliers
$121.00/1g

88246-12-2
8 suppliers
inquiry
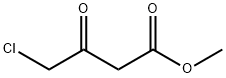
32807-28-6
256 suppliers
$10.00/5g
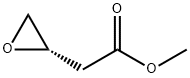
127001-17-6
0 suppliers
inquiry
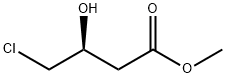
86728-93-0
78 suppliers
$55.00/250mg

88246-12-2
8 suppliers
inquiry

67-56-1
736 suppliers
$9.00/25ml
201230-82-2
1 suppliers
inquiry

556-52-5
251 suppliers
$17.67/1gm:

88246-12-2
8 suppliers
inquiry