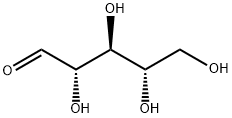
Ribose synthesis
- Product Name:Ribose
- CAS Number:24259-59-4
- Molecular formula:C5H10O5
- Molecular Weight:150.13
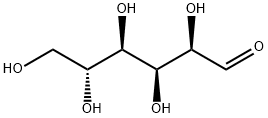
1500092-25-0
0 suppliers
inquiry

50-69-1
676 suppliers
$5.00/5g

24259-59-4
360 suppliers
$5.00/1g
50-99-7
986 suppliers
$6.00/25g

921-60-8
255 suppliers
$32.00/250mg
Yield:-
Reaction Conditions:
with trifluoroacetic acid in water at 120; for 4 h;
Steps:
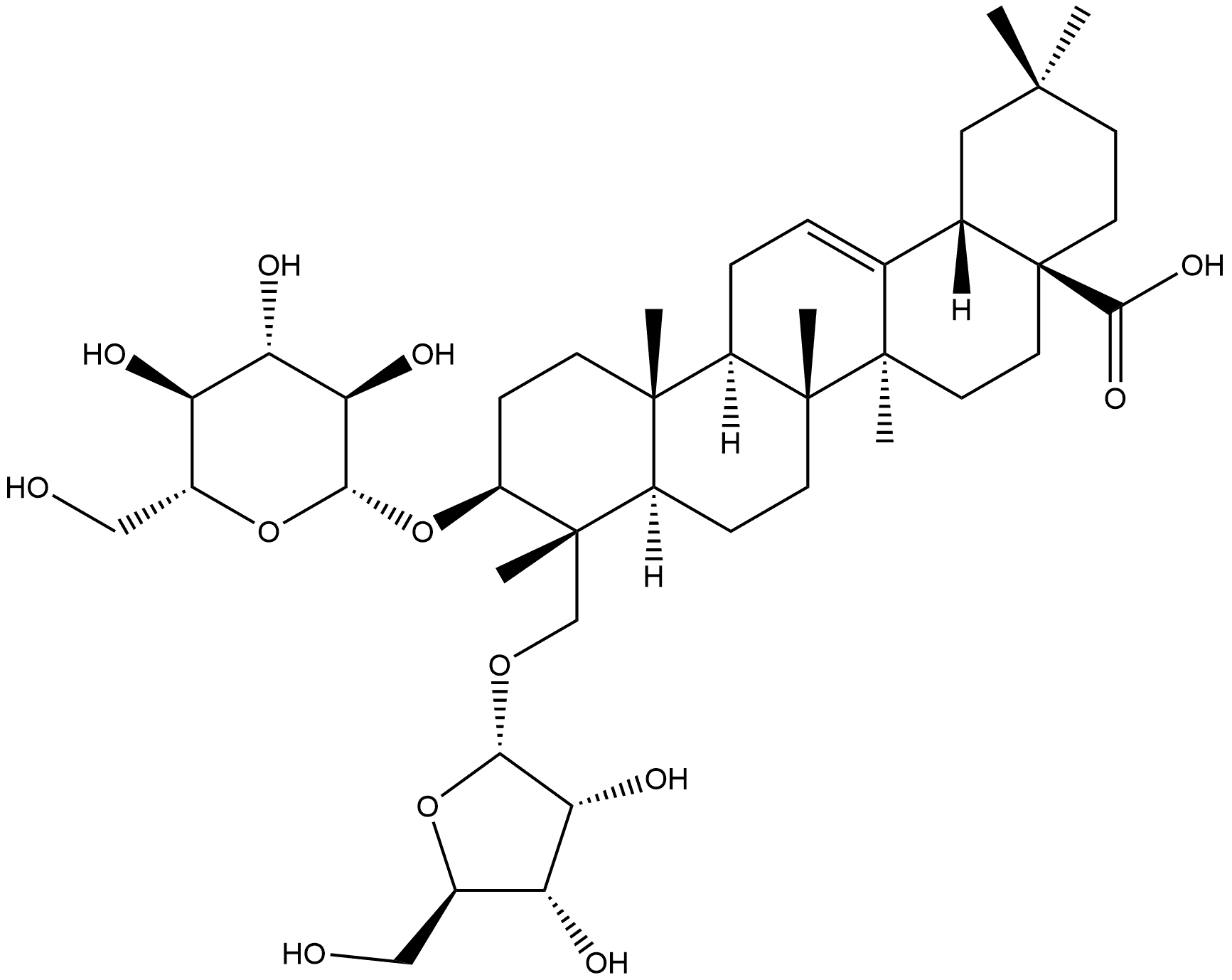
Acid hydrolysis of compound 1
Compound 1 (about 4 mg) was added into a solution of water (1 mL) and 2N aqueous CF3COOH(2 mL), heated to 120°C under reflux conditions for 4 h. The mixture was diluted with water (2 mL) and then extracted with EtOAc (3 £2 mL). The combined organic phase was washed with brine and evaporated to dryness to afford the aglycones. The aqueous phase was concentrated. Then dry pyridine (1 mL) and L-cysteine methyl ester hydrochloride (2 mg) were added into the residue. Each mixture was reacted at 60°C for 1 h, and 0.5mL of (trimethylsilyl) imidazole dissolved in H2O was added, followed by heating to dryness at 60°C for 2 h. Each dried reactant was extracted with n-hexane (3 £ 1 mL) and H2O (1 mL, each). The n-hexane fraction was subjected to GC (column: Rtx-1, 0.25mm i.d. 0.25 mm, length 30 m). The conditions of GC were flame ionization detector; column temperature 100-180°C (10°C min-1)and 180-230°C (3°C min21); injector temperature 250°C; detector temperature 300°C and the carrier gas (N2, 0.8mLmin21). Under these conditions, these sugars of each reactants were identified by comparison with authentic samples: tR (min) 7.56 (D-ribose), 7.85 (L-ribose), 10.49 (D-glucose) and 11.10 (L-glucose).
References:
Shu, Zhan;Chen, Zhong;Liu, Yan-Li;Zhu, Wei-Feng;Feng, Yu-Lin;Xu, Qiong-Ming;Li, Xiao-Ran;Yang, Shi-Lin [Natural Product Research,2013,vol. 27,# 23,p. 2196 - 2201]
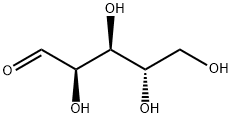
5328-37-0
462 suppliers
$5.00/1g

24259-59-4
360 suppliers
$5.00/1g

5328-37-0
462 suppliers
$5.00/1g
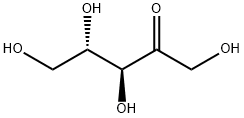
2042-27-5
42 suppliers
$50.00/25mg

24259-59-4
360 suppliers
$5.00/1g
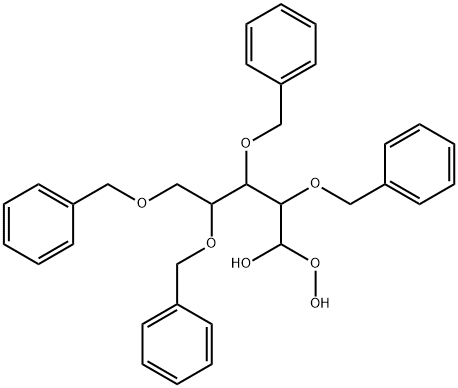
110773-08-5
0 suppliers
inquiry

24259-59-4
360 suppliers
$5.00/1g
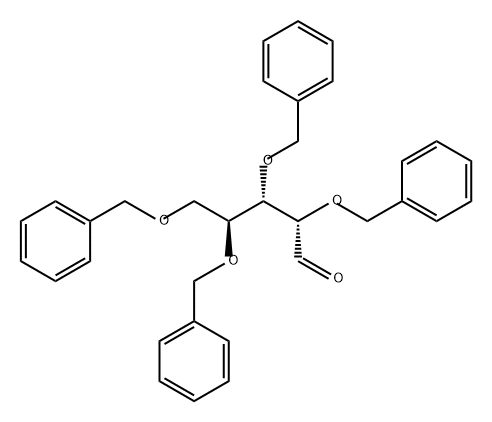
110744-96-2
0 suppliers
inquiry

24259-59-4
360 suppliers
$5.00/1g