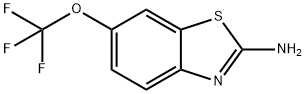
Riluzole synthesis
- Product Name:Riluzole
- CAS Number:1744-22-5
- Molecular formula:C8H5F3N2OS
- Molecular Weight:234.2
461-82-5
448 suppliers
$10.00/1g
333-20-0
430 suppliers
$13.50/100G

1744-22-5
391 suppliers
$5.00/250mg
Yield:1744-22-5 94%
Reaction Conditions:
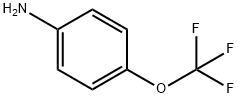
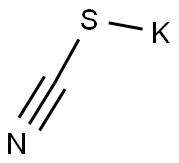
Stage #1:4-(trifluoromethoxy)aniline;potassium thioacyanate in acetic acid at 20; for 0.333333 h;
Stage #2: with bromine in acetic acid at 20;
Steps:
1.2. General procedure for the synthesis of 6-(trifluoromethoxy)benzo[d]thiazol-2-amine (1)
Potassium thiocyanate (40 mmol)was added to a solution of 4-(trifluoromethoxy)aniline (10 mmol)in glacial acetic acid (20 mL). After the mixture stirring for 20 minat r. t., the reaction was cooled down just to keep the solution liquid. The solutionof bromine (15 mmol) in glacial acetic acid (5 mL) was added dropwiseover the 30 min. The reaction was allowed to reach r. t. and stirred overnight.The mixture was diluted with H2O, alkalized with Na2CO3and extracted with ethyl acetate (3 x 50 mL). The combined organiclayers were dried over Na2SO4, filtered and the solventwas under reduced pressure to dryness. The residue was recrystallized from diethylether-petrol ether to yield 6-(trifluoromethoxy)benzo[d]thiazol-2-amine as light-yellow solid(94%). 1H and 13C NMR, melting point and analytical data werein agreement with those reported in literature.
References:
Hroch, Lukas;Benek, Ondrej;Guest, Patrick;Aitken, Laura;Soukup, Ondrej;Janockova, Jana;Musil, Karel;Dohnal, Vlastimil;Dolezal, Rafael;Kuca, Kamil;Smith, Terry K;Gunn-Moore, Frank;Musilek, Kamil [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 15,p. 3675 - 3678] Location in patent:supporting information

461-82-5
448 suppliers
$10.00/1g
3674-54-2
76 suppliers
$40.00/5g

1744-22-5
391 suppliers
$5.00/250mg

133840-98-9
11 suppliers
$395.00/1 G

1744-22-5
391 suppliers
$5.00/250mg
142229-74-1
81 suppliers
$15.00/1g

1744-22-5
391 suppliers
$5.00/250mg

461-82-5
448 suppliers
$10.00/1g

1744-22-5
391 suppliers
$5.00/250mg