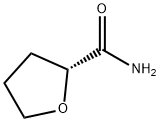
(S)-TETRAHYDROFURAN-2-CARBONITRILE synthesis
- Product Name:(S)-TETRAHYDROFURAN-2-CARBONITRILE
- CAS Number:164472-78-0
- Molecular formula:C5H7NO
- Molecular Weight:97.12

164472-76-8
0 suppliers
inquiry

164472-78-0
12 suppliers
$205.00/1G
Yield:164472-78-0 57%
Reaction Conditions:
with silver perchlorate in dichloromethane at 20; for 1 h;
Steps:
4.1.7. (R)-Tetrahydrofuran-2-carbonitrile (6a)24
AgClO4 (175 mg, 0.844 mmol) was added to compound (R)-5a (136 mg, 0.766 mmol) in dry CH2Cl2 (5 mL). The mixture was stirred at room temperature for 1 h. Brine was added, the phases were separated, and the aqueous phase was extracted with CH2Cl2. The combined organic phases were dried over MgSO4 and the solvents evaporated in vacuo. The crude product was purified by flash chromatography (petroleum ether/Et2O 4:1, Rf=0.44) to give (R)-6a with <5% impurities (42.7 mg, 0.440 mmol, 57%, 98% ee) as a colorless oil. -34.2 (c 1.0, CHCl3); lit.:1 -26.1 (c 0.85, CHCl3, 92% ee); 1H NMR (500 MHz, CDCl3): δ=4.71 (dd, J=7.1, 4.6 Hz, 1H), 3.92-4.02 (m, 2H), 2.21-2.31 (m, 2H), 2.09-2.19 (m, 1H), 1.97-2.05 (m, 1H); 13C NMR (500 MHz, CDCl3): δ=119.5, 69.2, 66.4, 31.8, 25.0.
References:
Hertzberg, Robin;Widyan, Khalid;Heid, Berenice;Moberg, Christina [Tetrahedron,2012,vol. 68,# 37,p. 7680 - 7684] Location in patent:experimental part
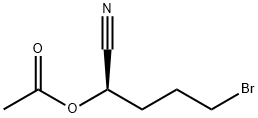
1393708-93-4
0 suppliers
inquiry

164472-78-0
12 suppliers
$205.00/1G