
Sacubitril Sodium synthesis
- Product Name:Sacubitril Sodium
- CAS Number:149690-05-1
- Molecular formula:C24H28NNaO5
- Molecular Weight:433.4726

108-30-5
685 suppliers
$5.00/25g
149690-12-0
239 suppliers
$48.00/1g

149690-05-1
97 suppliers
inquiry
Yield:149690-05-1 100 g
Reaction Conditions:
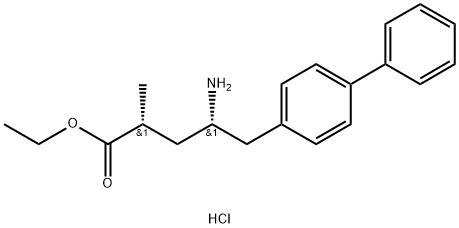
Stage #1: succinic acid anhydride;(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methylpentanoic acid ethyl ester hydrochloridewith triethylamine in dichloromethane at 0 - 35; for 3 h;
Stage #2: with sodium carbonate in dichloromethane;water at 25 - 42; for 9 h;
Steps:
2 EXAMPLE 2: (0215) Preparation of sacubitril sodium of Formula Π:
Compound of formula rV (100 g), succinic anhydride (34.5 gms) and dichloromethane (800 ml) were added into a round bottom flask at 25-35°C and stirred for 30 mins and then cooled to 0-6°C. To the reaction mixture, Triethylamine (80 ml) was added lot wise over 3 hrs in 2 lots by maintaining the temperature below 10°C. After completion of the reaction by HPLC, dilute hydrochloric acid (35 ml in 310 ml water) was added to the above reaction mass. The temperature of the reaction mass was raised to 25-35°C and the layers were separated. The aq layer was extracted with dichloromethane and combined the organic layers and washed with 10% sodium chloride solution. Water (30 ml) and sodium carbonate (19.8 gms) were added to the resulting product containing organic layer at 25-35°C and stirred for 3 hrs. The temperature of the reaction mixture was raised to 38-42°C_and maintained for 6h at the same temperature. After the completion of the reaction, the excess water was removed by azeotropic distillation and then the cooled to 30°C, filtered, washed with dichloromethane and distilled the solvent completely. The crude reaction mass was stripped off with methyl ethyl ketone (200 mL) and degassed for 30 min at 50°C. HPLC analysis revealed the content of lactam impurity of Formula C: about 1 to 1.5%; succinamide Impurity of Formula D: about 0.09%; HPLC purity: 98.62%. (0217) Methyl ethyl ketone (500 ml) was added to the above crude material at 50-55°C and stirred for 10-20 min. n-Heptane (1000 mL) was added to the above reaction mass at the same temperature and maintained for 6 hrs. The reaction mass was then cooled to 25-35°C, maintained for 2 hrs, filtered, washed with a mixture of MEK: n-Heptane (1:2, 75mL). The wet material was dried initially at 25-35°C for 2hrs and then dried at 47-53°C for 8hrs to obtain sacubitril sodium as white color solid; yield- 100 gms; HPLC purity 99.7%, desethyl sacubitril of Formula B: 0.02%, lactam impurity of Formula C: 0.06%; succinamide impurity of Formula D: Nil.
References:
WO2018/69833,2018,A1 Location in patent:Page/Page column 29

149709-62-6
267 suppliers
$39.00/1mg

149690-05-1
97 suppliers
inquiry
![Ethyl (2R,4S)-4-([1,1'-biphenyl]-4-ylmethyl)-2-methyl-4-(2,5-dioxopyrrolidin-1-yl)butanoate](/CAS/20180703/GIF/1038924-97-8.gif)
1038924-97-8
66 suppliers
inquiry

149690-05-1
97 suppliers
inquiry

149690-12-0
239 suppliers
$48.00/1g

149690-05-1
97 suppliers
inquiry
![(2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoic acid](/CAS/20150408/GIF/1012341-50-2.gif)
1012341-50-2
425 suppliers
$5.00/100mg

149690-05-1
97 suppliers
inquiry