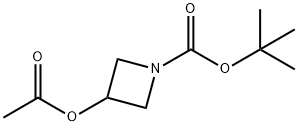
tert-Butyl 3-acetoxyazetidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-acetoxyazetidine-1-carboxylate
- CAS Number:1215205-53-0
- Molecular formula:C10H17NO4
- Molecular Weight:215.25

108-24-7
5 suppliers
$14.00/250ML
141699-55-0
384 suppliers
$6.00/1g

1215205-53-0
26 suppliers
inquiry
Yield:1215205-53-0 98%
Reaction Conditions:
with dmap;N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20; for 16 h;
Steps:
1-84-1
Preparation Example 1-84-13-Acetoxy-azetidine-1-carboxylic acid tert-butyl ester 0.27 g (1.559 mmol) of the compound obtained from Preparation Example 1-83-2, 0.61 g (4.677 mmol) of diisopropylethylamine and a catalytic amount of 4-dimethylaminopyridine were dissolved in 7 mL of dichloromethane and cooled to 0° C. To the solution was added 0.32 g (2 mmol) of acetic anhydride and stirred at room temperature for 16 hours. The reaction mixture was distilled in vacuo to remove a solvent and purified by column chromatography using a mixed solution of hexane and ethyl acetate in the ratio of 3:1 to obtain the title compound 0.33 g (98%).1H NMR (400 MHz, CDCl3); δ 5.12 (1H, m), 4.23 (2H, dd), 4.12 (2H, dd), 2.09 (3H, s), 1.44 (9H, s)
References:
US2011/166121,2011,A1 Location in patent:Page/Page column 56

141699-55-0
384 suppliers
$6.00/1g

1215205-53-0
26 suppliers
inquiry

127-08-2
622 suppliers
$6.00/25g
254454-54-1
247 suppliers
$15.00/1g

1215205-53-0
26 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G

1215205-53-0
26 suppliers
inquiry