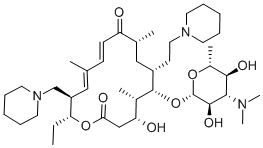
Tildipirosin synthesis
- Product Name:Tildipirosin
- CAS Number:328898-40-4
- Molecular formula:C41H71N3O8
- Molecular Weight:734.02
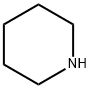
110-89-4
3 suppliers
$15.96/100ML
66767-27-9
0 suppliers
inquiry

328898-40-4
180 suppliers
$50.00/25mg
Yield:328898-40-4 43.78%
Reaction Conditions:
Stage #1: (11E,13E)-(4R,5S,6S,7R,9R,15R,16R)-6-((2R,3R,4S,5S,6R)-4-Dimethylamino-3,5-dihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-16-ethyl-4-hydroxy-7-(2-hydroxy-ethyl)-15-hydroxymethyl-5,9,13-trimethyl-oxacyclohexadeca-11,13-diene-2,10-dionewith 1-methyl-1H-imidazole;triphenylphosphine in dichloromethane at -5 - 0; for 0.666667 h;
Stage #2: with iodine in dichloromethane at -5 - 5; for 8 h;
Stage #3: piperidinewith potassium carbonate in dichloromethane at 45; for 5 h;Reagent/catalyst;
Steps:
9.3; 9.4; 9.5
3. Dissolve 100g of intermediate in 600mL of dichloromethane at low temperature -5-0 °C, add 50g of triphenylphosphine and 62g of methylimidazole under stirring, stir for 40min, slowly add 100g of iodine in 10 times, keep The temperature in the reaction system is not higher than 5°C, after the addition, maintain the low temperature -5-0 °C reaction for 8h, after the reaction is completed,The obtained mixture was quenched by adding 650 mL of an aqueous Na2SO3 solution.Stir for 30 min, let stand for layering, and separate the organic layer.240 g of piperidine and 420 g of K2CO3 were added to the organic layer, and stirring was started.After heating to 45 ° C and refluxing for 5 h, after the completion of the reflux, the resulting mixed system was filtered to remove K 2 CO 3 , 500 mL of pure water was added to the filtrate, stirred for 30 min, allowed to stand for separation, separated, and the aqueous layer was dehydrated to obtain an organic phase;4. Add 500 mL of pure water to the organic phase, adjust the pH to 3 with 1500 mL of 2 mol/L hydrochloric acid solution, stir for 30 minutes, let stand for stratification, separate the aqueous layer, and cool the aqueous layer to 0 ° C with 6 mol / LNaOH solution adjusts the pH to 8,The mixture was allowed to stand for stratification, and the aqueous phase was separated. The aqueous phase was extracted twice with 500 mL of dichloromethane. The organic layer was combined, dried over anhydrous Na?Concentrated to dryness, get 72.8g20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide solid crude;5. Dissolve the crude 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide in acetonitrile, place it in the refrigerator, and precipitate solids until the precipitate is complete. Filter and filter the cake. The mixture was rinsed with cold acetonitrile and dried under vacuum overnight at 40 ° C to afford 62.4 g of 20,23-dipiperidinyl-5-O-mycaminosyl-tylorolide as a white solid. Total yield: 43.78%; color: white; purity: 96.37%.
References:
CN108264530,2018,A Location in patent:Paragraph 0078; 0082; 0083; 0090; 0092; 0093; 0097; 0098

110-89-4
3 suppliers
$15.96/100ML

1003024-02-9
0 suppliers
inquiry

328898-40-4
180 suppliers
$50.00/25mg
1003024-00-7
9 suppliers
inquiry

328898-40-4
180 suppliers
$50.00/25mg