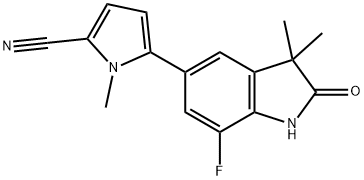
WAY-255348 synthesis
- Product Name:WAY-255348
- CAS Number:872141-23-6
- Molecular formula:C16H14FN3O
- Molecular Weight:283.3
Yield:872141-23-6 84.3%
Reaction Conditions:
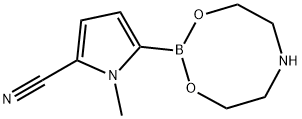
Stage #1: diethanolamine complex of 5-cyano-1-methyl-1H-pyrrol-2-ylboronic acid;5-bromo-7-fluoro-3,3-dimethyl-1,3-dihydro-2H-indol-2-onewith sodium carbonate;bis-triphenylphosphine-palladium(II) chloride in tetrahydrofuran;water at 65; for 2 h;
Stage #2: with N-acetylcystein in tetrahydrofuran at 65; for 1 h;
Steps:
3
Example 3 Preparation of 5-(7-Fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile; A mixture of 5-bromo-7-fluoro-3,3-dimethyl-1,3-dihydro-indol-2-one (100 g, 0.387 mole), 5-[1,3,6,2]Dioxazaborocan-2-yl-1-methyl-1H-pyrrole-2-carbonitrile (110 g, 0.252 mole), sodium carbonate (Na2CO3; 49.2 g, 0.233 mol), THF (2 L), water (0.5 L), and PdCl2(PPh3)2(2.72 g, 3.9 mmol) was heated to 65° C. for 2 hours. The solution was then cooled to room temperature and a saturated sodium chloride solution (1.0 L) was added. The organic phase was then isolated, N-acetyl-L-cysteine (31.7 g, 0.194 mol) was added, the mixture was heated to 65° C. for 1 hour and then cooled to room temperature. The cooled mixture was filtered through a glass-sintered funnel packed with the Celite reagent (100 g) and charcoal (50 g). The filtrate was concentrated by distillation to a volume of about 300 mL and ethanol (EtOH; 500 mL) was added to the slurry. The mixture was distilled to a volume of about 400 mL and then cooled to about 20 to about 25° C. The solid was filtered, washed with ethanol, and dissolved in acetone (2.0 L) at 55° C. The resultant solution was filtered at about 40 to about 50° C. and then concentrated to a volume of about 200 mL. EtOH (1.0 L) was added and the mixture was concentrated to a volume of about 400 mL. The concentrated mixture was then cooled to about 5° C., the precipitated solid filtered, washed with ethanol and dried through vacuum to give a white solid (92.7 g, 84.3% yield with 99% HPLC purity).
References:
US2007/27327,2007,A1 Location in patent:Page/Page column 8; 10