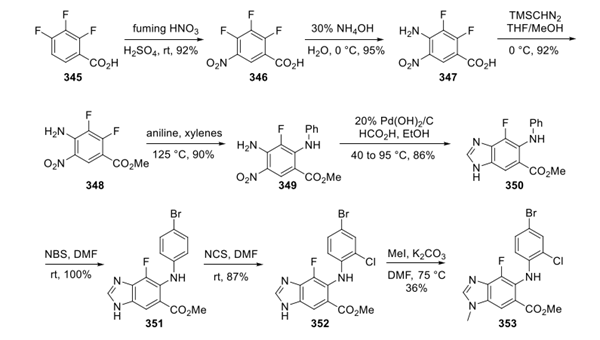
Synthesis of Surufatinib
Surufatinib is synthesised from dichloropyrimidine and hydroxyindole by substitution reaction. This process requires the participation of Surufatinib Amine.
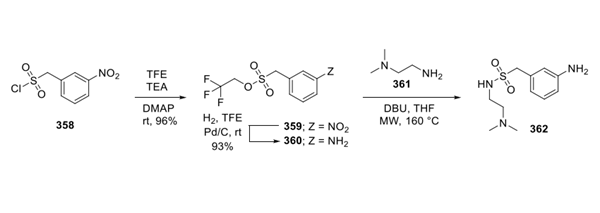
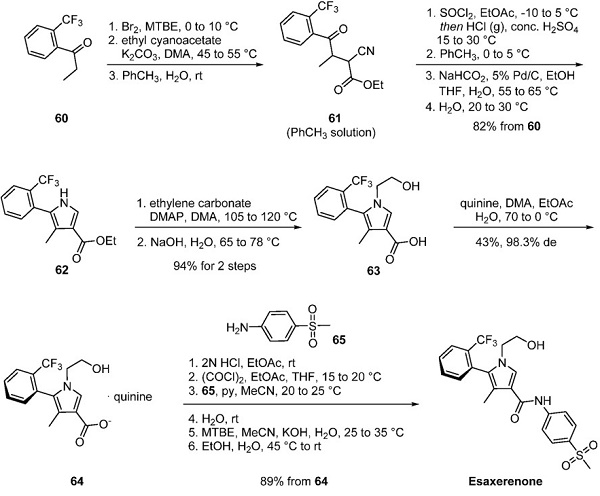
Jan 5,2024 InhibitorsThe Synthesis method of Esaxerenone
Esaxerenone, a novel, nonsteroidal, selective mineralocorticoid receptor antagonist (MRA) discovered by Exelixis and developed by Daiichi Sankyo, was approved in Japan to treat hypertension.
Jan 5,2024 APIHow is Selumetinib Sulfate Synthesised?
Selumetinib Sulfate was prepared by a two-step chemical reaction by first synthesising Selumitinib Benzimidazole intermediates using 2,3,4-trifluorobenzoic acid as starting material.
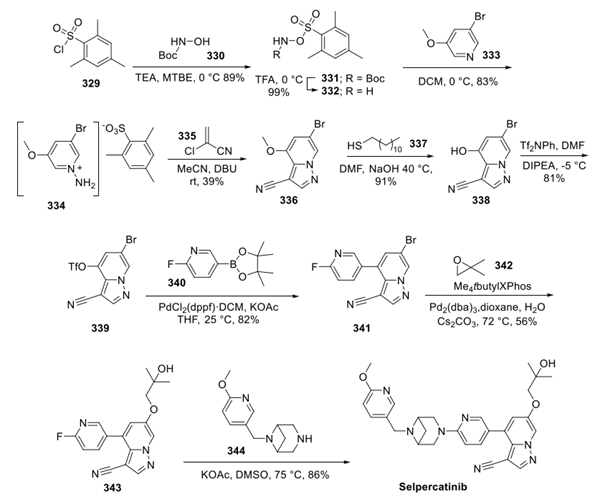
Jan 5,2024 InhibitorsSelpercatinib: Synthesis and Description
Selpercatinib (LOXO-292) is synthesised using sulfonyl chloride as a raw material by chemical reaction.
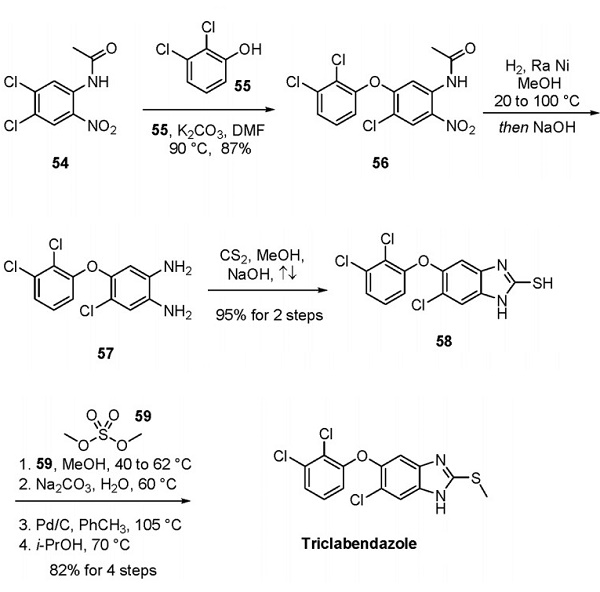
Jan 5,2024 InhibitorsA brief introduction to triclabendazole
Triclabendazole, also known as CGA 89317 or CGP 23030, is an orally bioavailable anthelmintic against infections caused by Fasciola species.
Jan 5,2024 APIHow is Sacituzumab Govitecan Synthesised?
The overall synthetic strategy for the preparation of sacituzumab govitecan inherently consists of three separate sequential components, construction of the payload (SN-38) followed by appendage with
Jan 5,2024 Drugsβ-Carotene: Dietary Sources and Benefits
β-Carotene is found in richer sources, mostly in brightly coloured fruits and vegetables such as carrots, spinach, kale, tomatoes, sweet potatoes, broccoli, onions, peppers, cantaloupe and apricots.
Jan 5,2024 Vitamins and Minerals medicinesHow to synthesize Relebactam?
Relebactam, formerly MK-7655, is a DBO that promises to contribute to the renaissance in antimicrobial chemotherapy.
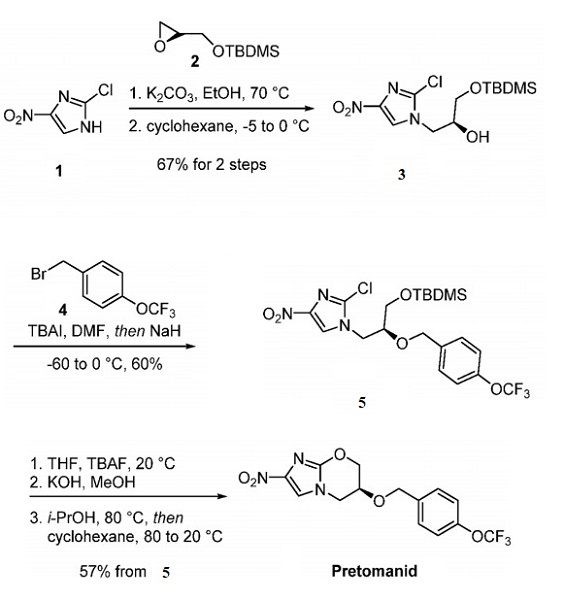
Jan 5,2024 APIThe Synthesis method and Pharmacokinetics of Pretomanid (PA 824)
Pretomanid (also known as PA 824) is a nitroimidazooxazine antimycobacterial drug with a complex mechanism of action.
Jan 5,2024 APIA semisynthetic pleuromutilin antibiotic: Lefamulin
Lefamulin, a semisynthetic pleuromutilin antibiotic, has been approved by the FDA for IV and oral treatment of community-acquired bacterial pneumonia (CABP) in adults.
Jan 5,2024 API