| Identification | More | [Name]
Benzaldehyde | [CAS]
100-52-7 | [Synonyms]
AKOS BBS-00003184
ARTIFICIAL ESSENTIAL OIL OF ALMOND
BALD
BENZALDEHYDE
BENZENECARBONAL
BENZOIC ALDEHYDE
BENZYL ALDEHYDE
BITTER ALMOND OIL
FEMA 2127
LABOTEST-BB LT00939687
OIL OF BITTER ALMOND
phenylmethanal
Almond artificial essential oil
almondartificialessentialoil
Artifical essential oil of almond
Artificial Almond Oil
Artificial bitter almond oil
artificialalmondoil
Benzaldehyde FFC
benzaldehydeffc | [EINECS(EC#)]
202-860-4 | [Molecular Formula]
C7H6O | [MDL Number]
MFCD00003299 | [Molecular Weight]
106.12 | [MOL File]
100-52-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Benzaldehyde is a clear to yellowish liquid
with an almond odor. The Odor Threshold is 0.042 ppm. | [Melting point ]
-26 °C (lit.) | [Boiling point ]
178-179 °C (lit.) | [density ]
1.044 g/cm 3 at 20 °C(lit.) | [vapor density ]
3.7 (vs air)
| [vapor pressure ]
4 mm Hg ( 45 °C)
| [FEMA ]
2127 | [refractive index ]
n20/D 1.545(lit.)
| [Fp ]
145 °F
| [storage temp. ]
Store below +30°C. | [solubility ]
H2O: soluble100mg/mL | [form ]
neat | [pka]
14.90(at 25℃) | [color ]
Pale yellow | [Odor]
Like almonds. | [PH]
5.9 (1g/l, H2O) | [PH Range]
5.9 | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, reducing agents, steam. Air, light and moisture-sensitive. | [biological source]
synthetic | [explosive limit]
1.4-8.5%(V) | [Odor Type]
fruity | [Water Solubility ]
<0.01 g/100 mL at 19.5 ºC | [FreezingPoint ]
-56℃ | [Sensitive ]
Air Sensitive | [JECFA Number]
22 | [Merck ]
14,1058 | [BRN ]
471223 | [Dielectric constant]
17.8(20℃) | [Cosmetics Ingredients Functions]
FRAGRANCE
DENATURANT
PERFUMING
FLAVOURING | [InChIKey]
HUMNYLRZRPPJDN-UHFFFAOYSA-N | [LogP]
1.4 at 25℃ | [Uses]
Benzaldehyde is a flavoring agent which is liquid and colorless, and
has an almond-like odor. it has a hot (burning) taste. it is oxidized
to benzoic acid when exposed to air and deteriorates under light.
it is miscible in volatile oils, fixed oils, ether, and alcohol; it is spar-
ingly soluble in water. it is obtained by chemical synthesis and by
natural occurrence in oils of bitter almond, peach, and apricot
kernel. it is also termed benzoic aldehyde. | [CAS DataBase Reference]
100-52-7(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzaldehyde(100-52-7) | [EPA Substance Registry System]
100-52-7(EPA Substance) | [CAS Number Unlabeled]
100-52-7 |
| Hazard Information | Back Directory | [Chemical Properties]
Benzaldehyde is a colorless to yellow, oily liquid with an odor of bitter almonds.
Benzaldehyde is commercially available in two grades: (i) pure benzaldehyde and (ii) and
double-distilled benzaldehyde. The latter has applications in the pharmaceutical, perfume,
and fl avor industries. Benzaldehyde may contain trace amounts of chlorine, water, benzoic acid, benzyl chloride, benzyl alcohol, and/or nitrobenzene. Benzaldehyde is ignited
relatively easily on contact with hot surfaces. This has been attributed to the property of
very low auto-ignition temperature. Benzaldehyde also undergoes autoxidation in air and
is liable to self-heat. Benzaldehyde exists in nature, occurring in combined and uncombined forms in many plants. Benzaldehyde is also the main constituent of the essential oils obtained by pressing the kernels of peaches, cherries, apricots, and other fruits.
Benzaldehyde is released into the environment in emissions from combustion processes,
such as gasoline and diesel engines, incinerators, and wood burning. It is formed in the
atmosphere through photochemical oxidation of toluene and other aromatic hydrocarbons.
Benzaldehyde is corrosive to gray and ductile cast iron (10% solution), and all concentrations of lead. However, pure benzaldehyde is not corrosive to cast iron. Benzaldehyde does
not attack most of the common metals, like stainless steels, aluminum, aluminum bronze,
nickel and nickel-base alloys, bronze, naval brass, tantalum, titanium, and zirconium. On
decomposition, benzaldehyde releases peroxybenzoic acid and benzoic acidBenzaldehyde is used in perfumes, soaps, foods, drinks, and other products; as a solvent for oils, resins, some cellulose ethers, cellulose acetate, and cellulose nitrate. The uses
of benzaldehyde in industries are extensive. For instance, in the production of derivatives that are employed in the perfume and fl avor industries, like cinnamaldehyde, cinnamyl alcohol, cinnamic acid, benzylacetone, and benzyl benzoate, in the production of
triphenylmethane dyes and the acridine dye, benzofl avin; as an intermediate in the pharmaceutical industry, for instance, to make chloramphenicol, ephedrin, and ampicillin,
as an intermediate to make benzoin, benzylamine, benzyl alcohol, mandelic acid, and
4-phenyl-3-buten-2-one (benzylideneacetone), in photochemistry, as a corrosion inhibitor
and dyeing auxiliary, in the electroplating industry, and in the production of agricultural
chemicals | [General Description]
A clear colorless to yellow liquid with a bitter almond odor. Flash point near 145°F. More denser than water and insoluble in water. Hence sinks in water. Vapors are heavier than air. The primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways. Used in flavoring and perfume making. | [Reactivity Profile]
A nontoxic, combustible liquid, reacts with oxidizing reagents. BENZALDEHYDE(100-52-7) must be blanketed with an inert gas at all times since BENZALDEHYDE(100-52-7) is oxidized readily by air to benzoic acid [Kirk-Othmer, 3rd ed., Vol. 3, 1978, p. 736]. In contact with strong acids or bases BENZALDEHYDE(100-52-7) will undergo an exothermic condensation reaction [Sax, 9th ed., 1996, p. 327]. A violent reaction was observed on contact with peroxyacids (peroxyformic acid) [DiAns, J. et al., Ber., 1915, 48, p. 1136]. An explosion occurred when pyrrolidine, BENZALDEHYDE(100-52-7), and propionic acid were heated to form porphyrins. | [Air & Water Reactions]
Oxidizes in air to form benzoic acid, which is moderately toxic by ingestion. Insoluble in water. | [Hazard]
Highly toxic. | [Health Hazard]
Exposures to theExposures to the vapor of benzaldehyde cause irritation to the upper respiratory tract,
intolerable irritation of the nose and throat, headache, nausea, dizziness, drowsiness, and
confusion. It is a CNS depressant. Exposures to benzaldehyde cause moderate to severe
eye irritation and prolonged periods of exposure cause corrosive effects to the skin, like
burns, scarring, and skin injury, fatigue, headache, nausea, dizziness, and loss of coordination. At higher concentrations, benzaldehyde produces more severe effects, such as
sore throat, abdominal pain, nausea, CNS depression, convulsions, and respiratory failure.
The estimated lethal dose of benzaldehyde has been reported as 2 oz. There is no data on
humans and the information and conclusions are based on evidence obtained from animal
studies. vapor of benzaldehyde cause irritation to the upper respiratory tract, | [Health Hazard]
Inhalation of concentrated vapor may irritate eyes, nose and throat. Liquid is irritating to the eyes. Prolonged contact with the skin may cause irritation. | [Potential Exposure]
In manufacture of perfumes, dyes, and
cinnamic acid; as solvent; in flavors. | [Fire Hazard]
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN1990 Benzaldehyde, Hazard class: 9; Labels:
9—Miscellaneous hazardous material. | [Incompatibilities]
The substance reacts with air, forming
explosive peroxides. Reacts violently with performic acid,
oxidants, aluminum, iron, bases, and phenol, causing fire
and explosion hazard. May self-ignite if absorbed in combustible
material with large surface area, or otherwise dispersed
over large areas. Reacts with rust, amines, alkalies, strong
bases, reducing agents such as hydrideds and active metals. | [Waste Disposal]
Incineration; add combustible
solvent and spray into incinerator with afterburner. | [Occurrence]
Present as cyanuric glucoside (amygdalin) in bitter almond, peach, apricot kernel and other Prunus
species; amygdalin is also present in various parts of the following plants: Sambucus nigra, Chrysophyllum arlen, Anacyclus
officinarnm, Anacyclus pedunculatus, Davallia brasiliensis, Lacuma deliciosa, Lacuma multiflora and others; free benzaldehyde has been reported found in several essential oils: hyacinth, citronella, orris, cinnamon, sassafras, labdanum and
patchouli. Reported found in strawberry jam, leek (raw) (Allium porrum L.), crispbread, Camembert, Gruyere de Comte,
provolone cheeses, black tea, salted and pickled plum, cooked trassi, Bantu beer, red sage (Texas sage) (S. coccinea Juss. Ex
Murr.), arrack, scallop, hog plum (Spondias mombins L.), chekur (Alpinia sessilis Kon. = Kaemferia galanga) and other natural
sources. | [Definition]
A
yellow organic oil with a distinct almondlike
odor. Benzenecarbaldehyde undergoes
the reactions characteristic of aldehydes
and may be synthesized in the laboratory
by the usual methods of aldehyde synthesis.
It is used as a food flavoring and in the
manufacture of dyes and antibiotics, and
can be readily manufactured by the chlorination
of methylbenzene and the subsequent
hydrolysis of (dichloromethyl)
benzene:
C6H5CH3 + Cl2 →C6H5CHCl2
C6H5CHCl2 + 2H2O →C6H5CH(OH)2+ 2HCl
C6H5CH(OH)2 →C6H5CHO + H2O. | [Preparation]
Benzaldehyde is prepared by hydrolysis of benzal chloride, for example, in acidic media in the presence of a catalyst such as ferric chloride or in alkaline media with aqueous sodium carbonate. Part of the commercially available benzaldehyde originates from a technical process for phenol. In this process, benzaldehyde is a by-product in the oxidation, in air, of toluene to benzoic acid. | [Aroma threshold values]
Detection: 100 ppb to 4.6 ppm; Recognition: 330 ppb to 4.1 ppm. | [Taste threshold values]
Taste characteristics at 50 ppm: sweet, oily, almond, cherry, nutty and woody | [Synthesis Reference(s)]
Chemical and Pharmaceutical Bulletin, 12, p. 403, 1964
The Journal of Organic Chemistry, 58, p. 4732, 1993 DOI: 10.1021/jo00069a043
Synthetic Communications, 16, p. 43, 1986 DOI: 10.1080/00397918608057686 | [Flammability and Explosibility]
Nonflammable | [Chemical Reactivity]
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Pharmacology]
Benzaldehyde significantly inhibited peptic activity in artificial gastric juice in vitro
(20-45% inhibition) and in vivo to the extent of 87% in normal healthy persons and ulcer patients
(Kleeberg, 1959). As a freshly prepared 1:500 solution, it exerted a marked antispasmodic effect,
relaxing the tonus and inhibiting contractions of various isolated smooth muscles of dog, cat, rat,
rabbit, mouse, guinea-pig, pig and frog and of a few human tissues. Injected into rabbits and other
animals it produced a marked relaxation of the intestines and urinary bladder and marked vasodilation
of the splanchnic vessel. Injection of 4 ml of a 5% solution iv into a cat caused a fall in
blood pressure and slowing of respiration. In dogs, 1 ml injected iv or sc or 2 ml/kg given orally
produced only a slight slowing of respiration. Injection of larger doses iv produced only a drop
in blood pressure, slight slowing of respiration and inhibition of intestinal contractions, with vasodilation
of the splanchnic vessel. In rabbits, iv injection of 20 ml of a 0-2% solution did not produce
dangerous results. Large injected doses of benzaldehyde exert their mosjt important toxic effects
on the medulla, with slowing or paralysis of respiration. In the intact animal, the heart is very
little affected; but benzaldehyde acts as a muscular depressant on isolated frog heart (Macht, 1922).
Treatment of isolated rat striated muscle for 1-5 min with 30 mM-benzaldehyde increased the
rate of propagation of contractures and the rate of structural breakdown of injured striated muscle
fibres. After more prolonged application (for 30 min), the rapid propagation of contracture continued
but the structural breakdown was inhibited (Busing, 1972).
Benzaldehyde possessed definite local anaesthetic properties in the sciatic nerves of cats, dogs
and frogs, in the eyes of rabbits and dogs (accompanied by irritation) and in the skin of frogs,
but was considered unsuitable for practical use because of its rapid oxidation to benzoic acid (Macht,
1922).
In a study of the toxic effects of cherry laurel water on mice and on isolated rat intestine, benzaldehyde
was found to aid in the detoxication of HCN by the formation of C6H5?CH(OH)?CN (Lanza
& Conte, 1964).
Benzaldehyde did not act as a cross-linking (tanning) agent for corium and aorta, since in a
015 M solution it did not increase the observed in vitro hydrothermal shrinkage temperatures of
goat skin and human, bovine and canine aortae (Milch, 1965).
The intestinal absorption-rate coefficients of benzaldehyde and related compounds were determined
by perfusion of aqueous solutions through the small intestines of anaesthetized rats (Nogami, Hanano
& Yamada, 1968).
No changes in gastric motor patterns, including gastric motility, were observed in rats after inhalation
of "toxic levels" (not specified) of benzaldehyde from a liquid sample placed in a test chamber
using recirculated air, or from a saturated paper applied to the trachea (Roth & Tansy, 1972).
Benzaldehyde in a concentration of 0-1 mmol/litre caused a 16% depression of the frequency of
electric-organ discharge in the mormyrid electric fish Gnathonemus moori (Walsh & Schopp, 1966). | [Synthesis]
Natural benzaldehyde is obtained by extraction and subsequent fractional distillation from botanical sources; synthetically, from benzyl chloride and lime or by oxidation of toluene | [Metabolism]
Benzaldehyde was among 300 volatile constituents detected in the urine of ten adults . It is commonly converted to hippuric acid in vivo. In the rabbit and dog, hippuric acid appears to be the only metabolite there being practically no formation of benzoyl glucuronide. The conversion of benzaldehyde to benzoic acid in the rabbit follows first-order reaction kinetics | [Purification Methods]
To diminish its rate of oxidation, benzaldehyde usually contains additives such as hydroquinone or catechol. It can be purified via its bisulfite addition compound but usually distillation (under nitrogen at reduced pressure) is sufficient. Prior to distillation it is washed with NaOH or 10% Na2CO3 (until no more CO2 is evolved), then with saturated Na2SO3 and H2O, followed by drying with CaSO4, MgSO4 or CaCl2. [Beilstein 7 IV 505.] | [Toxics Screening Level]
The initial risk screening level ( IRSL) for benzaldehyde is 0. 4 μg/m3 based on an annual averaging time. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S24:Avoid contact with skin . | [RIDADR ]
UN 1990 9/PG 3
| [WGK Germany ]
1
| [RTECS ]
CU4375000
| [F ]
8 | [Autoignition Temperature]
374 °F | [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
29122100 | [storage]
Benzaldehyde should be kept stored in a tightly closed container and protected against
physical damage. Storage of the chemical substance outside or in a detached area is preferred, whereas inside storage should be in a standard flammable liquids storage room or
cabinet. Benzaldehyde should be kept separated from oxidizing materials. Also, storage
and use areas should be no smoking areas. Containers of this material may be hazardous
when empty since they retain product residues (vapors, liquid); observe all warnings and
precautions listed for the product | [Precautions]
Workers should be careful when using benzaldehyde because there is a risk of spontaneous combustion. It may ignite spontaneously if it is absorbed onto rags, cleaning cloths,
clothing, sawdust, diatomaceous earth (kieselguhr), activated charcoal, or other materials with large surface areas in workplaces. Workers should avoid handling the chemical
substance and should not cut, puncture, or weld on or near the container. Exposure of
benzaldehyde to air, light, heat, hot surfaces such as hot pipes, sparks, open flames, and
other ignition sources should be avoided. Workers should wear proper personal protective
clothing and equipment | [Safety Profile]
Poison by ingestion
and intraperitoneal routes. Moderately
toxic by subcutaneous route. An allergen.
Acts as a feeble local anesthetic. Local
contact may cause contact dermatitis.
Causes central nervous system depression in
small doses and convulsions in larger doses.
A skin irritant. Questionable carcinogen
with experimental tumorigenic data.
Mutation data reported. Combustible liquid.
To fight fire, use water (may be used as a
blanket), alcohol, foam, dry chemical. A
strong reducing agent. Reacts violently with
peroxyformic acid and other oxidizers. See
also ALDEHYDES. | [Hazardous Substances Data]
100-52-7(Hazardous Substances Data) | [Toxicity]
LD50 in rats, guinea pigs (mg/kg): 1300, 1000 orally (Jenner) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Toluene-->Sodium carbonate-->Palladium-->Chlorine-->Benzyl chloride-->Zinc oxide-->CARBON MONOXIDE-->Aluminium chloride hexahydrate-->Benzyl alcohol-->Molybdenum trioxide-->Ozone-->trans-Cinnamaldehyde-->Zinc phosphate-->Cinnamon oil-->Amygdalin | [Preparation Products]
2,3,5-Triphenyltetrazolium chloride-->whitener WG for wool-->Benzalacetone-->3,5-DIPHENYLPYRAZOLE-->Epalrestat-->Bis(dibenzylideneacetone)palladium-->2-[2-(4-Fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxo-N-phenylpentanamide-->L-Arginine hydrochloride-->2-(Acetylamino)-3-phenyl-2-propenoic acid--> Methyl indole-2-carboxylate-->TRANS-2-PHENYL-1-CYCLOPROPANECARBOXYLIC ACID-->1-AMINO-4-METHYLPIPERAZINE DIHYDROCHLORIDE MONOHYDRATE-->Acid Blue 90-->Diaveridine-->Nifedipine-->Reactive Blue 104-->3,4-Dichlorobenzylamine-->Tris(dibenzylideneacetone)dipalladium-->Nitrotetrazolium blue chloride-->BENZYLHYDRAZINE DIHYDROCHLORIDE-->(R)-(+)-N-Benzyl-1-phenylethylamine-->2-((E)-2-Hydroxy-3-phenylacryloyl)benzoic acid ,97%-->(E)-3-Benzylidene-3H-isochromene-1,4-dione ,97%-->Reactive Blue BRF-->FLAVANONE-->L-Phenylglycine-->Benzenemethanol, ar-methyl-, acetate-->ASTRAZON BRILLIANT RED 4G-->2-amino-5-chloro-diphenyl methanol-->Magentagreencrystals-->Acid Blue 9-->alpha-Hexylcinnamaldehyde-->DL-Mandelic acid-->N,N'-BISBENZYLIDENEBENZIDINE-->2,4,5-Triphenylimidazole-->4-Hydroxybenzylideneacetone-->5,5-Diphenylhydantoin-->Propafenone Hydrochloride-->N,N'-Dibenzyl ethylenediamine diacetate-->2-PHENYL-1.3-DIOXOLANE-4-METHANOL |
| Questions And Answer | Back Directory | [description]
Benzaldehyde is an organic compound, and is synthetized by the way that the hydrogen of benzene is substituted by aldehyde. It is the most simple, and also the most commonly used industrial aromatic aldehyde. It is a colorless liquid at room temperature and has a special almond odor. Benzaldehyde is a compound that aldehyde is directly linked to the phenyl group, because it has a similar bitter almond flavor. Benzaldehyde widely exists in plant, especially in the Rosaceae plants. It is mainly in the form of glycosides in plant stem bark, leaves or seeds, such as amygdalin, bitter almond, cherry, laurel, peach. Benzaldehyde is naturally in bitter almond oil, patchouli oil, hyacinth oil, cananga oil. The compound is also in the nutlets and nuts, and exists in the form of Amygdalin, which is combination of glycosides. The chemical properties of Benzaldehyde is similar to that of aliphatic aldehydes, but It is also different. Benzaldehyde cannot reduce fehling reagent. When the reducing fat is used to reduce the benzaldehyde, the main products are benzene methanol, four substituted for the ortho-glycol and two-phenyl ethylene glycol. In the presence of potassium cyanide, two molecules of benzaldehyde form benzoin by acceptance the hydrogen atom. The substitution reaction in aromatic nucleus of benzaldehyde is mainly the meta-position product. For example, the main product is the m-nitrobenzaldehyde , when benzaldehyde is nitrated.
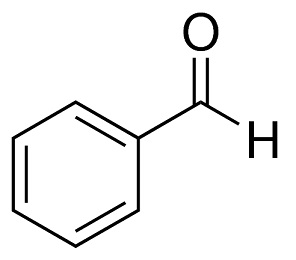
benzaldehyde structure
| [Chemical Properties]
Benzaldehyde is the main, characteristic component of bitter almond oil. It occurs in many other essential oils and is a colorless liquid with a bitter almond odor. In the absence of inhibitors, benzaldehyde undergoes autoxidation to perbenzoic acid, which reacts with a second molecule of benzaldehyde to yield benzoic acid. Hydrogenation of benzaldehyde yields benzyl alcohol, and condensation with aliphatic aldehydes leads to additional fragrance substances or their unsaturated intermediates.Unsaturated araliphatic acids are obtained through the Perkin reaction, for example, the reaction with acetic anhydride to give cinnamic acid.
Benzaldehyde is used in aroma compositions for its bitter almond odor. It is the starting material for a large number of araliphatic fragrance and flavor materials.
| [Uses]
1. Benzaldehyde is an important raw material for medicine, dyestuff, perfume and resin industry. It also can be used as solvent, plasticizer and low temperature lubricant. In essence, it is mainly used for the deployment of food flavor. A small amount of benzaldehyde is daily use in flavor and flavor of tobacco. In spite of being widely used as commercial food condiment and industrial solvents, the main use of benzyl alcohol is still used to synthesize a variety of other compounds from pharmaceuticals to plastic additives. Benzyl alcohol is an important intermediate product in the production of perfumes, spices, and some aniline dyes.
Mandelic acid was synthesized by benzaldehyde as the starting reagent: With the first hydrocyanic acid reacts with benzaldehyde, then mandelonitrile hydrolyzed to Racemic mandelic acid. Glacialist LaChepelle and Stillman reported Ice crystallization is inhibited by benzaldehyde and aldehydes ice in 1966, so as to prevent the thick frost formation (Depth Hoar). This process can prevent snowslide caused by the instability of the snow cover. However, this compound has not been used extensively, because of the destruction of vegetation and polluted water sources.
2.It is mainly used for the preparation of flavors, such as almond, cherry, peach, nuts, etc., the amount is up to 40%. As aromatizing agent canned cherry syrup, adding amount is sugar 3mL/kg.
3. Pharmaceutical, dyestuff, spice intermediates. For the production of oxygen based benzene formaldehyde, lauric acid, lauric aldehyde, malachite, benzyl benzoate, benzyl aniline and benzylidene acetone etc.. Used to tune the soap flavor, edible essence, etc.
4. As the head of the special aroma, it is used trace formula for fragrance, such as lilac, white, violet, jasmine, acacia, sunflower, sweet plum, orange flower, Tofu pudding etc.. Also it is used in soap. Also it can be used as edible spices for almond, coconut cream, berries, cherries, apricots, peaches, plums, walnuts, and vanilla bean, spicy flavor. Wine with flavors such as rum, brandy, etc.
5. Benzaldehyde is an intermediate of herbicide resistance, plant growth regulator, and anti-amine.
6. Used as a reagent for the determination of ozone, phenol, alkaloid and methylene. Used in the preparation of spices.
| [Production]
Benzaldehyde can be prepared by a variety of ways.- Obtained from natural essential oils by fractionation.
- Ozone oxidation and thiourea reduction reaction of natural cinnamon oil (containing cinnamaldehyde constituent 80% or more)
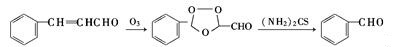 - Catalytic oxidation of Toluene
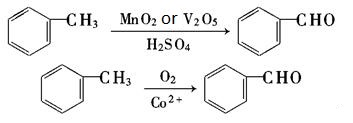 - Hydrolyze dichloromethane under alkaline conditions.
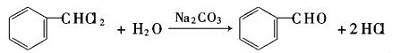
| [reactions]
Benzaldehyde can be slowly oxidized to benzoic acid in air, so a small amount of hydroquinone is often added to prevent its oxidation.
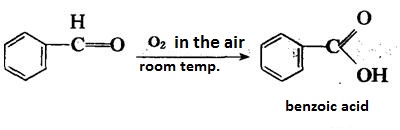
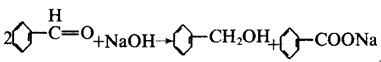
There is no α-H atom in the benzaldehyde molecule. Disproportionation reaction(Cannizarro reaction) may occur under the action of concentrated alkali:
Heating benzaldehyde in the presence of catalyst of cyanide ion, it will occur bimolecular condensation:
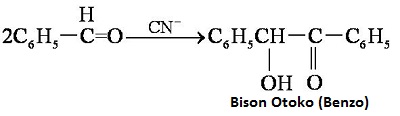
|
|
|