Lobeglitazone Sulfate Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Lobeglitazone sulfate, an oral peroxisome proliferator-activated
receptor (PPARa/c) dual agonist with IC50 = 20 and 18 nM respectively,
was developed by Chong Kun Dang Pharmaceutical in Korea
for the treatment of diabetes. This drug is differentiated from
two other PPAR agonists available—pioglitazone and rosiglitazone
—which lack PPARa activity. The most likely processscale
preparation of lobeglitazone sulfate follows the route
described in a process communication from Chong Kun Dang
Pharmaceutical.
Synthese
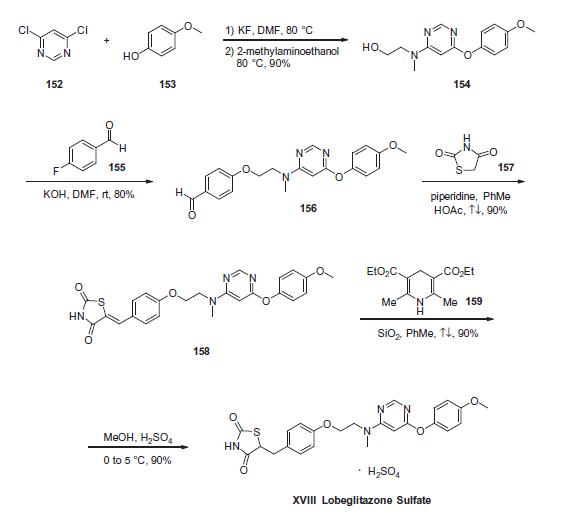
Commercially available 4,6-dichloropyrimidine (152) was treated
with a stoichiometric equivalent of p-methoxyphenol (153) in
the presence of KF in warm DMF . Upon completion of
this reaction, 2-methylaminoethanol was added to the mixture to
provide pyrimidine 154 in high yield. Next, alcohol 154 underwent
a substitution reaction with p-fluorobenzaldehyde (155)
under basic conditions to provide alkoxy benzaldehyde 156 which
was converted to the benzylidene thiazolidindione 158 upon subjection
to Knoevenagel conditions with 2,4-thiazolidinedione (157)
in 90% yield. Finally, reduction of olefin 158 was facilitated by
treatment with the Hantzsch ester (159) in the presence of silica
gel followed by treatment with methanolic sulfuric acid (96%) at
low temperature to ultimately furnish lobeglitazone sulfate in
90% yield.
Lobeglitazone Sulfate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

