Chlormethylpivalat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R10:Entzündlich.
R20/21/22:Gesundheitsschädlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-Sätze Betriebsanweisung:
S16:Von Zündquellen fernhalten - Nicht rauchen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S41:Explosions- und Brandgase nicht einatmen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Chemische Eigenschaften
Clear colorless to slightly yellow liquid
Verwenden
Chloromethyl pivalate was used in the synthesis of pivaloyloxy methyl ester of ofloxacin as prodrug. It was used as the reagent during the synthesis of an isoindoline-annulated, tricyclic sultam library via microwave-assisted, continuous-flow organic synthesis.
Application
Chloromethyl pivalate is a pharmaceutical intermediate compound used in the synthesis of active pharmaceutical ingredients. It is also involved in the acylation reaction with 9-(2-phosphonomethoxyethyl)adenine. It is used as a protecting agent for the N-protection of amines. It is also used in the preparation of thivaloyloxymethyl ester of ofloxacin as a prodrug. It is also used in the preparation of sulbactam pivoxil by reaction with the sodium salt of sulbactam. Besides, it is used as a reagent in the synthesis of isoindoline cyclic and continuous flow organic synthesis.
Allgemeine Beschreibung
Chloromethyl pivalate reacts with sodium salt of sulbactam to yield sulbactam pivoxil. It undergoes acylation reaction with 9-(2-phosphonylmethoxyethyl)adenine (PMEA) to yield
bis(pivaloyloxymethyl) PMEA.
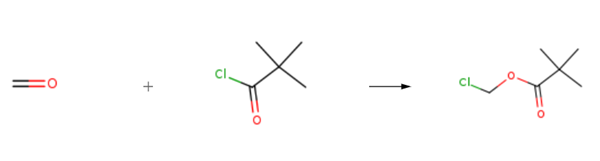
Synthese
Chloromethyl pivalate is prepared by the reaction of formaldehyd and pivaloyl chloride. The specific synthesis steps are as follows:
A mixture of pivaloyl chloride (8.56 g, 71 mmol), paraformaldehyde (2.13 g, 71 mmol) and zinc chloride (75 mg, 0.55 mmol) was stirred at 80 °C for 2 h. Purification by vacuum distillation afforded chloromethyl pivalate (41) as a colourless oil (6.29 g, 44.7 mmol, 59%). bp 80 °C/15 mmHg [lit.1 bp 80-81 °C/15 mmHg]; 1H NMR (400 MHz, CDCl3) δ 1.24 (9H, s, tBu), 5.72 (2H, s, CH2).
Chlormethylpivalat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

