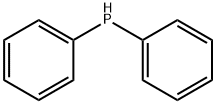
Diphenylphosphine
- CAS No.
- 829-85-6
- Chemical Name:
- Diphenylphosphine
- Synonyms
- HPPh2;PH2PH;Diphenylphosphin;98% Ph2PH;AURORA KA-1107;DIPHENYLPHOSPHINE;Diphenyl phospine;Diphenyl-phosphane;PHOSPHINE, DIPHENYL;DPP/DIPHENYLPHOSPHIN
- CBNumber:
- CB0720195
- Molecular Formula:
- C12H11P
- Molecular Weight:
- 186.19
- MDL Number:
- MFCD00003040
- MOL File:
- 829-85-6.mol
- MSDS File:
- SDS
| Melting point | -14.5 °C |
|---|---|
| Boiling point | 280 °C(lit.) |
| Density | 1.07 g/mL at 25 °C(lit.) |
| vapor pressure | 2 mm Hg ( 110 °C) |
| refractive index |
n |
| Flash point | -18°C (Hexane) |
| storage temp. | 2-8°C |
| solubility | Chloroform |
| form | liquid |
| pka | diphenylphosphine is weakly basic. The pKa of the protonated derivative is 0.03. |
| color | colorless |
| Specific Gravity | 0.68 |
| Water Solubility | Miscible with ethanol, ether, benzene, concentrated hydrochloric acid. Immiscible with water. |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 742504 |
| InChIKey | GPAYUJZHTULNBE-UHFFFAOYSA-N |
| CAS DataBase Reference | 829-85-6(CAS DataBase Reference) |
| FDA UNII | F9B5T7O7ZY |
| NIST Chemistry Reference | Phosphine, diphenyl-(829-85-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H250-H315-H319-H335 | |||||||||
| Precautionary statements | P210-P222-P231+P232-P302+P352-P305+P351+P338-P370+P378 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 17-36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | UN 2845 4.2/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 8-10-13-23 | |||||||||
| HazardClass | 4.2 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
Diphenylphosphine price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 252964 | Diphenylphosphine 98% | 829-85-6 | 10g | $85.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 252964 | Diphenylphosphine 98% | 829-85-6 | 1kg | $1450 | 2024-03-01 | Buy |
| Alfa Aesar | 056169 | Diphenylphosphine | 829-85-6 | 10g | $71.19 | 2023-01-06 | Buy |
| Alfa Aesar | 056169 | Diphenylphosphine | 829-85-6 | 50g | $278.25 | 2023-01-06 | Buy |
| Strem Chemicals | 15-1700 | Diphenylphosphine, 99% | 829-85-6 | 10g | $68 | 2024-03-01 | Buy |
Diphenylphosphine Chemical Properties,Uses,Production
Organophosphorus compound
Diphenylphosphine is commonly used in laboratory as organic phosphorus compound, it is unpleasant odor colorless liquid, and it is pungent, it is easily oxidized in air and can cause spontaneous combustion, it is sensitive to air and light, it need the protection of nitrogen. It can be used as precursor to synthesize some organic phosphine ligand. By deprotonation, it can be converted to diphenylphosphine compound: Ph2PH + nBuLi → Ph2PLi + nBuH, such as 1,2-bis (diphenylphosphino) ethane and 1,3-bis (diphenylphosphino) propane phosphine ligands etc, Wittig-Horner reagents and the synthesis of quaternary phosphonium salt is usually by means of diphenylphosphino alkylation to achieve.
Diphenylphosphine such as sodium and lithium diphenylphosphine and diphenylphosphine compound can add to carbon heteroatom double bond as nucleophile. For example, in the condition of concentrated hydrochloric acid at 100℃, diphenylphosphine can add to aldehyde carbon atoms of benzaldehyde: Ph2PH + PhCHO → Ph2P (O) CH2Ph, when compares with tertiary phosphine, alkalinity of diphenylphosphine is weaker. The pKa of conjugate acid diphenylphosphine is 0.03: Ph2PH2 + → Ph2PH + H +
Preparation: Lithium diphenylphosphine can generated by inexpensive triphenylphosphine and the with the cancellation of water can obtain diphenylphosphine: (1) PPh3 + 2 Li → LiPPh2 + LiPh (2) LiPPh2 + H2O → Ph2PH + LiOH.
The above information is edited by the chemicalbook of Wang Xiaodong.
Uses
It can be used the intermediates of organic, catalysts.
Chemical Properties
Diphenylphosphine is an organophosphorus compound commonly used in laboratories. It is a clear colorless to slightly yellow liquid with unpleasant odor, irritating, easily oxidized in air and spontaneously combusts, sensitive to air and light, and needs to be protected by nitrogen. It can be used as a precursor for the synthesis of a variety of organophosphine ligands. These ligands, in turn, are used in homogeneous catalysis for many applications including: asymmetric hydrogenation, coupling chemistry, ethylene oligomerization, hydroformylation, hydration of nitriles, and polymerization of alkenes.
Uses
Diphenylphosphine acts as an intermediate in the preparation of diphenylphosphide derivatives, phosphonium salts, phosphine ligands and Wittig-Horner reagents. The presence of hydrogen atom bonded to phosphorus undergoes Michael-like addition to activated alkenes. It is involved in the preparation of 1,2-bis(diphenylphosphino)ethane and (phenyl-(phenylmethyl)phosphoryl)benzene. Further, it is used in the synthesis of aminophosphines and chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
Uses
suzuki reaction
Uses
Diphenylphosphine is used in the synthesis of aminophosphines for application as catalysts. It is also used in the preparation of chiral palladacycles with N-heterocyclic carbene ligands as catalysts.
Preparation
Diphenylphosphine can be prepared from triphenylphosphine by reduction to lithium diphenylphosphide, which can be protonated to give the title compound:
PPh3 + 2 Li → LiPPh2 + LiPh
LiPPh2 + H2O → Ph2PH + LiOH
Diphenylphosphine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Puyang Huicheng Electronic Materials Co., Ltd. | +86-0393-+86-0393-8910800 +8615839383373 | info@huichengchem.com | China | 529 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18777 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| AB PharmaTech,LLC | 323-480-4688 | sales@acrospharmatech.com | United States | 989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29883 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
Related articles
- Diphenylphosphine: Applications in Organometallic Chemistry and Water Treatment
- Diphenylphosphine excels in catalysis by customizing reaction pathways and in water treatment by creating durable, high-capaci....
- Oct 24,2024
View Lastest Price from Diphenylphosphine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-05 | Diphenylphosphine
829-85-6
|
US $0.00-0.00 / kg | 1kg | 99% | 20ton | Shanghai Bojing Chemical Co.,Ltd. | |
 |
2023-08-14 | Diphenylphosphine
829-85-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-12-16 | Diphenylphosphine
829-85-6
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Weibang Biotechnology Co., Ltd |
-

- Diphenylphosphine
829-85-6
- US $0.00-0.00 / kg
- 99%
- Shanghai Bojing Chemical Co.,Ltd.
-

- Diphenylphosphine
829-85-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Diphenylphosphine
829-85-6
- US $0.00-0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
829-85-6(Diphenylphosphine)Related Search:
1of4