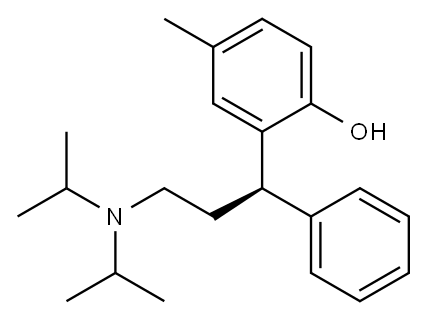
Tolterodine
- CAS No.
- 124937-51-5
- Chemical Name:
- Tolterodine
- Synonyms
- DETROL;TOLTERODINE L-TARTRATE;Kabi 2234;PNU-20058E;PNU 200583;DETRUSITOL;Tolterodine;Tolterodine-d6;TolterodineBase;Tolterodine-d14
- CBNumber:
- CB1128442
- Molecular Formula:
- C22H31NO
- Molecular Weight:
- 325.49
- MDL Number:
- MFCD00865264
- MOL File:
- 124937-51-5.mol
| alpha | D25 +72° (c = 1.0 in CH2Cl2) |
|---|---|
| Boiling point | 442.2±45.0 °C(Predicted) |
| Density | 1.003±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO: ≥20mg/mL |
| pka | pKa 9.8 (Uncertain) |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 124937-51-5(CAS DataBase Reference) |
| FDA UNII | WHE7A56U7K |
| ATC code | G04BD07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H361 |
| Precautionary statements | P281-P301+P310 |
| Hazard Codes | Xn,N |
| Risk Statements | 63-51/53 |
| Safety Statements | 22-36/37-57 |
| WGK Germany | 3 |
Tolterodine price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | T535795 | R-(+)-Tolterodine | 124937-51-5 | 5mg | $135 | 2021-12-16 | Buy |
| TRC | T535795 | R-(+)-Tolterodine | 124937-51-5 | 25mg | $540 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | D34369 | Tolterodine-d14HCl | 124937-51-5 | 1mg | $625 | 2021-12-16 | Buy |
| ChemScene | CS-1799 | Tolterodine 99.55% | 124937-51-5 | 50mg | $672 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004447 | TOLTERODINE 95.00% | 124937-51-5 | 1G | $675.15 | 2021-12-16 | Buy |
Tolterodine Chemical Properties,Uses,Production
Chemical Properties
Pale Yellow Gel
Originator
Detrol,Pharmacia and Upjohn
Uses
A muscarinic receptor antagonist. Used in the treatment of urinary incontinence.
Definition
ChEBI: Tolterodine is a tertiary amine. It has a role as a muscarinic antagonist, a muscle relaxant and an antispasmodic drug. It is functionally related to a p-cresol.
Manufacturing Process
Trans-cinnamic acid (100 g, 675 mmol) is added to a 1 L 4-neck round bottom
flask equipped with a mechanical stirrer, thermocouple, and nitrogen inlet.
Para-cresol (76.6 g, 708 mmol) is preheated in a water bath at 60°C and
added to the cinnamic acid followed by concentrated sulfuric acid (13.0 ml,
243 mmol). The reaction is immediately heated to a set point of 122.5°C and
stirred at 120°-125°C until judged to be complete by HPLC analysis. When the
reaction is complete the mixture is cooled to 100°C and added to a
prewarmed separatory funnel (500 ml). The bottom layer containing the
sulfuric acid is removed and toluene (280 ml), water (50 ml) and potassium
carbonate (47%, 10 ml) are added to the separatory funnel containing the
crude product. The pH of the aqueous layer is adjusted to between 5-8 with
additional 47% potassium carbonate. The layers are separated and the
organic layer is then washed once with water (50 ml). The organic layer is
concentrated to a final volume of approximately 150 ml under reduced
pressure. Isopropanol (350 ml) is then added, and distillation is continued to
a volume of 350 ml. Isopropanol (150 ml) is again added and again distilled
to 350 ml (2 times). The mixture is then cooled to 30°-40°C with rapid
stirring until the product crystallizes. The rapid stirring is continued after
crystallization. The product is cooled to 0°-5°C and held at this temperature
for 1 h, filtered and washed with isopropanol (200 ml) cooled to 0°-5°C. If the
last portion of the wash is colored the wash is continued until no more color is
removed. The solids are then dried at 60°C under reduced pressure to give
the 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-one, melting point
(uncorrected) 83°-85°C.
3,4-Dihydro-6-methyl-4-phenyl-2H-benzopyran-2-one (100.0 g, 420.2 mmol)
is added to toluene (500 ml). The mixture is degassed by purging alternately
with vacuum and nitrogen and then cooled to -21°C. Diisobutylaluminum
hydride in toluene solution (DIBAL, 1.5 M, 290 ml, 435 mmol) is then slowly
added over 2 h via add funnel while maintaining the reaction temperature at -
20°-25°C. The reaction is usually done when the DIBAL add is completed. If the reaction is not done additional DIBAL can be added in increments. When
the reaction is done (<1% lactone) ethyl acetate (45 ml) is added at -20°-
25°C via add funnel. Very little exotherm is observed. Next, citric acid (23%,
500 ml) is added. The mixture is stirred at 45°-50°C for 1 h (or stirred
overnight at 20°-25°C), the phases are separated, the organic phase is
washed with water (2 times 300 ml). The organic phase is concentrated to
250 ml under reduced pressure. Methanol (500 ml) is added, and the mixture
is concentrated to 250 ml. The methanol addition and distillation is repeated
to give the 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol in methanol
solution.
3,4-Dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol in methanol (500 ml) is
slowly added to palladium on carbon (5%, 22 g, 1.5 mmol) while maintaining
a slight nitrogen purge. If 3,4-dihydro-6-methyl-4-phenyl-2H-benzopyran-2-ol
is added too quickly without a nitrogen purge the catalyst will ignite the
methanol. Diisopropylamine (147.0 ml, 1.05 mol) is added, and the mixture is
hydrogenated at 45-50 psi and 48°C until the reaction is judged to be
complete by HPLC (<2% lactol). The reaction is usually done after 10 h, but
can be run overnight. The reaction mixture is cooled and removed from the
hydrogenator using a methanol (150 ml) rinse. The combined reaction mixture
and rinse is filtered through a bed of solka floc (10 g). The solka floc is
washed thoroughly with methanol (100 ml) and the filtrate is concentrated to
remove methanol while ethyl acetate is being added back. The volume of this
solution of the (2-diisopropylamino)ethyl)benzyl)-p-cresol is adjusted to 700
ml using ethyl acetate and the mixture is heated to 55°C.
To form the hydrochloride salt of the (2-diisopropylamino)ethyl)benzyl)-p_x0002_cresol (Tolterodine), concentrated hydrochloric acid (52.5 ml, 630 mmol) is
added over 15 min. The resulting slurry is gradually cooled to -15°-20°C and
held at this temperature for 1 h. Tolterodine hydrochloride is collected by
filtration, washed 3 times with ethyl acetate, and dried overnight under
reduced pressure at 600 to give the tolterodine hydrochloride, melting point
199°-201°C.
Tolterodine hydrochloride (130.0 g, 359 mmol), methylene chloride (1.3 L)
and water (650 ml) are mixed. The mixture is stirred rapidly while adding
sodium hydroxide (50%, 13.0 ml) and sodium carbonate (13.0 g, 123 mmol).
The pH as determined by pH paper is 10-11. After stirring thoroughly for
approximately 15 min two clear homogeneous phases form. Stirring is
continued for another 45 min, the layers are separated and the organic phase
is washed with water (2 times 650 ml). The methylene chloride mixture is
concentrated under reduced pressure. The concentrate is dissolved in ethanol
(325 ml) and warmed to 60°-70°C. L-tartaric acid (80.84 g, 539 mmol)
slurried in hot ethanol (810 ml) is added via add funnel at 60°-70°C over
approximately 30 min. When the addition is done the slurry is refluxed for 1
h, gradually cooled to 0°C and held at this temperature for 1 h. The slurry is
filtered, washed with ethanol (2 times 260 ml) previously cooled to 0°C, and
dried overnight under reduced pressure at 60°C to give the crude title
compound. The crude product (136.0 g) and ethanol (5.44 L) are mixed and
heated to 80°C for 30 min. The mixture is concentrated to half the initial
volume by distilling 2.72 L of ethanol. The mixture is gradually cooled to 20°-
25°C over 1 h, placed in an ice bath, and held at 0°C for 1 h. The tolterodine
L-tartrate is collected by filtration, washed with ethanol (2 times 272 ml )
previously cooled to 0°C, and dried overnight under reduced pressure at 60°C to give product. This procedure was repeated a second time on 81.0 g of once
recrystallized tolterodine L-tartrate to give the optically active (+)-(R)-2-(α-
(2-(diisopropylamino)ethyl)benzyl)-p-cresol L-tartrate, melting point
(uncorrected)=210°-211°C.
Therapeutic Function
Anticholinergic
General Description
Tolterodine (Detrol), 2-[3-[bis(1-methylethyl)amino]-1-phenyl-propyl]-4-methyl-phenol, is an antimuscarinicagent that acts on M2 and M3 muscarinic subtypereceptors. By competitively blocking of the muscarinicreceptors results in a reduction of the smooth muscle tone,allowing for greater volume of urine to be stored in the bladder.This results in less urinary incontinence, urgency, andfrequency.
Tolterodine Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30240 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22883 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9639 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5978 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
View Lastest Price from Tolterodine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-27 | Tolterodine
124937-51-5
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Tolterodine
124937-51-5
|
US $30.00-56.00 / mg | 99.71% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-08-12 | Tolterodine
124937-51-5
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Tolterodine
124937-51-5
- US $0.00-0.00 / Kg/Drum
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Tolterodine
124937-51-5
- US $30.00-56.00 / mg
- 99.71%
- TargetMol Chemicals Inc.
-

- Tolterodine
124937-51-5
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
124937-51-5(Tolterodine)Related Search:
1of4