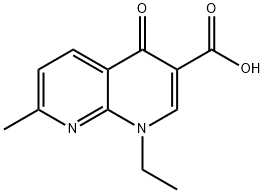
Nalidixic acid
- CAS No.
- 389-08-2
- Chemical Name:
- Nalidixic acid
- Synonyms
- neggram;Nalidixic acid BP93.CH.P.95;1,4-DIHYDRO-1-ETHYL-7-METHYL-4-OXO-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID;cybis;nalix;NOGRAM;uropan;uroneg;uroman;negram
- CBNumber:
- CB1194283
- Molecular Formula:
- C12H12N2O3
- Molecular Weight:
- 232.24
- MDL Number:
- MFCD00006884
- MOL File:
- 389-08-2.mol
| Melting point | 227-229 °C (lit.) |
|---|---|
| Boiling point | 374.4°C (rough estimate) |
| Density | 1.2243 (rough estimate) |
| refractive index | 1.6660 (estimate) |
| storage temp. | 2-8°C |
| solubility | chloroform: 20 mg/mL, clear |
| form | Powder |
| pka | pKa 6.11± 0.02(Approximate) |
| color | White to light yellow |
| Water Solubility | 0.1 G/L (23 ºC) |
| Merck | 14,6359 |
| BRN | 750515 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 389-08-2(CAS DataBase Reference) |
| FDA UNII | 3B91HWA56M |
| Proposition 65 List | Nalidixic Acid |
| ATC code | J01MB02 |
| EPA Substance Registry System | Nalidixic acid (389-08-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H351 | |||||||||
| Precautionary statements | P202-P264-P270-P280-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 63-42/43-40-20/21/22-22 | |||||||||
| Safety Statements | 22-36/37-45-24-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QN2885000 | |||||||||
| F | 8-10-23 | |||||||||
| HS Code | 29339190 | |||||||||
| Toxicity | LD50 in mice (mg/kg): 3300 orally; 500 s.c.; 176 i.v. (Lesher, 1962) | |||||||||
| NFPA 704 |
|
Nalidixic acid price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 97023 | Nalidixic acid analytical standard | 389-08-2 | 100mg | $72.6 | 2024-03-01 | Buy |
| TCI Chemical | N0490 | Nalidixic Acid >95.0%(T) | 389-08-2 | 25g | $63 | 2024-03-01 | Buy |
| Alfa Aesar | B25096 | Nalidixic acid, 99% | 389-08-2 | 5g | $30.6 | 2024-03-01 | Buy |
| Alfa Aesar | B25096 | Nalidixic acid, 99% | 389-08-2 | 25g | $98.5 | 2024-03-01 | Buy |
| Cayman Chemical | 19807 | Nalidixic Acid ≥98% | 389-08-2 | 10g | $32 | 2024-03-01 | Buy |
Nalidixic acid Chemical Properties,Uses,Production
Chemical Properties
Crystalline Powder
Originator
Neggram,Winthrop,US,1964
Uses
For the treatment of urinary tract infections caused by susceptible gram-negative microorganisms, including the majority of E. Coli, Enterobacter species, Klebsiella species, and Proteus species.
Uses
Quinolone antibacterial.
Uses
Nalidixic acid(NegGram) is a synthetic 1,8-naphthyridine antimicrobial agent with a limited bacteriocidal spectrum. It is an inhibitor of the A subunit of bacterial DNA gyrase. Evidence exists that the active metabolite, hydroxynalidixic acid, binds stron
Definition
ChEBI: A monocarboxylic acid comprising 1,8-naphthyridin-4-one substituted by carboxylic acid, ethyl and methyl groups at positions 3, 1, and 7, respectively.
Manufacturing Process
A warm solution containing 41 grams of 4-hydroxy-7-methyl-1,8-
naphthyridine-3-carboxylic acid and 39 grams of potassium hydroxide in 1
liter of ethanol and 200 cc of water was treated with 50 cc of ethyl iodide and
the resulting mixture was refluxed gently overnight, acidified with hydrochloric
acid and cooled. The resulting precipitate was collected and recrystallized
twice from acetonitrile to yield 26 grams (56% yield) of 1-ethyl-7-methyl-4-
oxo-1,8-naphthyridine-3-carboxylic acid, MP 229° to 230°C.
The starting material is prepared by reacting 2-amino-6-methylpyridine with
ethoxymethylene-malonic acid diethyl ester and then reacting that product
with sodium hydroxide.
brand name
Neggram(Sanofi Aventis).
Therapeutic Function
Antibacterial
Antimicrobial activity
It displays good activity in vitro against a wide range of Enterobacteriaceae.
General Description
Cream-colored powder.
Air & Water Reactions
Insoluble in water.
Health Hazard
SYMPTOMS: Ingestion of Nalidixic acid may cause nausea, vomiting, abdominal pain, allergic reactions and possible liver damage.
Fire Hazard
Flash point data for Nalidixic acid are not available, but Nalidixic acid is probably combustible.
Pharmaceutical Applications
A 1,8 naphthyridone derivative available for oral administration.
Pharmacokinetics
Oral absorption: >90%
Cmax 1 g oral: c. 25 mg/L
Plasma half-life:c.1.5h
Volume of distribution :0.4 L/kg
Plasma protein binding: 93%
The plasma concentrations achieved after oral administration vary widely. In infants with acute shigellosis, absorption is much impaired by diarrhea. Administration with an alkaline compound leads to higher plasma concentrations, partly as the result of enhanced solubility (nalidixic acid is much more soluble at higher pH) and absorption and partly because of reduced tubular reabsorption.
It is rapidly metabolized, principally to the hydroxy acid, which is bacteriologically active, and glucuronide conjugates, which are not. The entire administered dose appears in the urine over a 24 h period. Elimination is reduced by probenecid. In the presence of renal impairment there is little accumulation of the active compound because it continues to be metabolized. However, elimination of metabolites is progressively delayed as renal function declines. About 4% of a dose appears in the feces.
Clinical Use
1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid (NegGram) occurs as a pale buff crystalline powder that is sparingly soluble in water and ether but solublein most polar organic solvents.Nalidixic acid is useful in the treatment of urinary tractinfections in which Gram-negative bacteria predominate.The activity against indole-positive Proteus spp. is particularlynoteworthy, and nalidixic acid and its congeners representimportant alternatives for the treatment of urinary tractinfections caused by strains of these bacteria resistant toother agents. Nalidixic acid is rapidly absorbed, extensivelymetabolized, and rapidly excreted after oral administration.The 7-hydroxymethyl metabolite is significantly more activethan the parent compound. Further metabolism of theactive metabolite to inactive glucuronide and 7-carboxylicacid metabolites also occurs. Nalidixic acid possesses at1/2elim of 6 to 7 hours. It is eliminated, in part, unchanged inthe urine and 80% as metabolites.
Side effects
Adverse reactions are generally those common to all quinolones: gastrointestinal tract and CNS disturbances and skin rashes, including eruptions related to photosensitivity. About half of the reported CNS reactions involve visual disturbances, hallucinations or disordered sensory perception. Severe excitatory states, including acute psychoses and convulsions, are usually observed in patients receiving high dosages. The drug should be avoided in patients with psychiatric disorders or epilepsy.
Acute intracranial hypertension has been observed in children, some of whom have also manifested cranial nerve palsies. Hemorrhage has occurred in patients who were also receiving warfarin, presumably due to displacement of the anticoagulant from its protein binding sites by the nalidixic acid. Hemolytic anemia has been described several times in infants with or without glucose-6-phosphate dehydrogenase deficiency; in adults, death has occurred from autoimmune hemolytic anemia. Arthralgia and severe metabolic acidosis have rarely been reported.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Human systemic effects: convulsions, hyperglycemia, sweating, and blood changes in children. Experimental reproductive effects.Questionable carcinogen with experimental carcinogenic and tumorigenic data. Human mutation data reported. Used as an antibacterial agent and urinary tract antiseptic. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
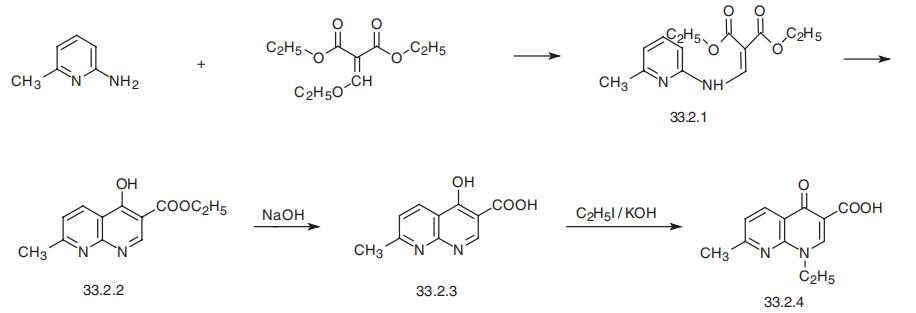
Nalidixic acid, 1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthiridin-3- carboxylic acid (33.2.4), is synthesized by the following scheme. In the first stage, the reaction of 2-amino-6-methylpyridine and diethyl ethoxymethylenemalonate forms the substituted product (33.2.1), which when heated cyclizes to ethyl ester of 4-hydroxy -7-methyl-1,8-napthiridin-3-carboxylic acid (33.2.2). Hydrolyzing the resulting product with a base gives the corresponding acid (33.2.3). Alkylating this with ethyl iodide in the presence of potassium hydroxide gives nalidixic acid.
Drug interactions
Potentially hazardous interactions with other drugs
Aminophylline and theophylline: possibly increased
risk of convulsions.
Analgesics: increased risk of convulsions with
NSAIDs.
Antibacterials: possibly antagonised by
nitrofurantoin.
Anticoagulants: anticoagulant effect of coumarins
enhanced.
Antimalarials: manufacturer of artemether with
lumefantrine advises avoid.
Ciclosporin: increased risk of nephrotoxicity.
Cytotoxics: increases risk of melphalan toxicity
Metabolism
Nalidixic acid is partially metabolised in the liver to hydroxynalidixic acid, which has antibacterial activity similar to that of nalidixic acid and accounts for about 30% of active drug in the blood. Both nalidixic acid and hydroxynalidixic acid are rapidly metabolised to inactive glucuronide and dicarboxylic acid derivatives; the major inactive metabolite 7-carboxynalidixic acid is usually only detected in urine.
Purification Methods
Nalidixic acid crystallises from H2O or EtOH as a pale buff powder. It is soluble at 23o in CHCl3 (3.5%), toluene (0.16%), MeOH (0.13%), EtOH (0.09%), H2O (0.01%) and Et2O (0.01%). It inhibits nucleic acid and protein synthesis in yeast. [Lesher et al. J Med & Pharm Chem 5 1063 1962.]
Nalidixic acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3958 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18779 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10319 | 58 |
Related articles
- Side-effects of Nalidixic acid
- Nalidixic acid (1-ethyl-l,4-dihydro-7-methyl-4-oxo-1, 8-naphthyridine- 3-carboxylic acid) is one of a series of 1,8-naphthyrid....
- Mar 11,2022
Related Qustion
- Q:What is nalidixic acid used for?
- A:Nalidixic acid has a bactericidal or bacteriostaic effect depending on the sensitivity of the microorganism and the concentrat....
- Sep 10,2024
View Lastest Price from Nalidixic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Nalidixic acid
389-08-2
|
US $45.00 / mg | 99.95% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Nalidixic acid
389-08-2
|
US $45.00 / mg | 99.95% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-10-25 | Nalidixic acid
389-08-2
|
US $0.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Nalidixic acid
389-08-2
- US $45.00 / mg
- 99.95%
- TargetMol Chemicals Inc.
-

- Nalidixic acid
389-08-2
- US $45.00 / mg
- 99.95%
- TargetMol Chemicals Inc.
-

- Nalidixic acid
389-08-2
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.