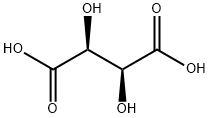
D-(-)-Tartaric Acid
- CAS No.
- 147-71-7
- Chemical Name:
- D-(-)-Tartaric Acid
- Synonyms
- TARTARIC ACID;(2S,3S)-2,3-DIHYDROXYSUCCINIC ACID;d-tartaric;D-TA;(S,S)-TARTARIC ACID;tartaricacidd-minus;(2S,3S)-2,3-Dihydroxybutanedioic acid;(2S,3S)-(-)-TARTARIC ACID;(2S,3S)-(-)-TARTARIC ACID FOR THE RESOLU;cas147-71-7
- CBNumber:
- CB3693922
- Molecular Formula:
- C4H6O6
- Molecular Weight:
- 150.09
- MDL Number:
- MFCD00004238
- MOL File:
- 147-71-7.mol
- MSDS File:
- SDS
| Melting point | 172-174 °C(lit.) |
|---|---|
| alpha | -12.1 º (c=20, H2O) |
| Boiling point | 191.59°C (rough estimate) |
| Density | 1,8 g/cm3 |
| refractive index | -12.5 ° (C=5, H2O) |
| Flash point | 210 °C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble100mg/mL, clear, colorless |
| form | Crystals or Crystalline Powder |
| pka | 3.0, 4.4(at 25℃) |
| color | White |
| Odor | odorless |
| optical activity | [α]20/D 13.5±0.5°, c = 10% in H2O |
| Water Solubility | 1394 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,9068 |
| BRN | 1725145 |
| Dielectric constant | 35.9(-10℃) |
| Stability | Stable. Incompatible with oxidizing agents, bases, reducing agents. Combustible. |
| InChIKey | FEWJPZIEWOKRBE-LWMBPPNESA-N |
| LogP | -1.081 (est) |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 147-71-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | RRX6A4PL3C |
| NIST Chemistry Reference | D-Tartaric acid(147-71-7) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dihydroxy-, (2S,3S)- (147-71-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318 | |||||||||
| Precautionary statements | P280-P305+P351+P338+P310 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36/37-37/39-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WW7875000 | |||||||||
| Autoignition Temperature | 425 °C | |||||||||
| Hazard Note | Light Sensitive | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29181200 | |||||||||
| NFPA 704 |
|
D-(-)-Tartaric Acid price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 95320 | D-(?)-Tartaric acid puriss., unnatural form, ≥99.0% (T) | 147-71-7 | 10g | $41 | 2024-03-01 | Buy |
| TCI Chemical | T0026 | D-(-)-Tartaric Acid >99.0%(T) | 147-71-7 | 25g | $45 | 2024-03-01 | Buy |
| TCI Chemical | T0026 | D-(-)-Tartaric Acid >99.0%(T) | 147-71-7 | 100g | $143 | 2024-03-01 | Buy |
| Alfa Aesar | A11264 | D-(-)-Tartaric acid, 99% | 147-71-7 | 25g | $40.2 | 2024-03-01 | Buy |
| Alfa Aesar | A11264 | D-(-)-Tartaric acid, 99% | 147-71-7 | 100g | $123 | 2024-03-01 | Buy |
D-(-)-Tartaric Acid Chemical Properties,Uses,Production
Chemical properties
There are three stereoisomers of tartaric acid: dextrose tartaric acid, levophyllic acid and meso tartaric acid. The optical rotation of the mixture of the same amount of dextrorotatory and levorotism is mutually offset, known as racemic tartaric acid. The mesomer does not exist in nature and can be synthesized chemically. Various tartaric acids are colorless crystals that are easily soluble in water.
Application
D-(-)-tartaric acid is widely used as an acidifier in beverages and food, similar to citric acid. It can also be used as a mordant for acid dye when combined with tannin. In the photographic industry, tartaric acid is used for some development and fixing processes. Its iron salts are photosensitive, making them suitable for blueprint production. Tartaric acid has the ability to form complexes with various metal ions, making it effective as a cleaning and polishing agent for metal surfaces. Potassium tartrate, known as Rochelle salt, is used to prepare Fehling reagent and is also employed as a laxative and diuretic in medicine. It is used as an intermediate for the synthesis of quinophan. Due to its piezoelectric properties, tartaric acid crystals find application in the electronics industry.
Preparation
D-(-)-tartaric acid is mainly present in the form of potassium salt in the fruit of a variety of plants, and a small amount of it exists in free form. We produce dextrose tartaric acid through glucose fermentation industrially. The racemate can be prepared by fumaric acid with potassium permanganate as oxidant. The mesomer can be prepared by maleic acid with potassium permanganate as oxidant. L-lactic acid can be obtained by resolution of racemates. In the practical application of tartaric acid, the main application is dextrose tartaric acid or its complex salt. The by-product tartra of brewing grape is the main raw material of actual production of tartaric acid, and the all tartaric acids are dextrose tartaric acids.
Description
D-Tartaric acid, also known as (S, S)-tartrate or D-threaric acid, belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. D-Tartaric acid has been detected but not quantified in loquats (Eriobotrya japonica). This could make D-tartaric acid a potential biomarker for consuming these foods. D-Tartaric acid is a secondary metabolite. Secondary metabolites are metabolically or physiologically non-essential metabolites that may serve as defense or signalling molecules. In some cases, they are molecules that arise from the incomplete metabolism of other secondary metabolites.
Chemical Properties
D-Tartaric acid is a white crystalline dicarboxylic acid found in many plants, particularly tamarinds and grapes. It is an acidulant that occurs naturally in grapes. It is hygroscopic and rapidly soluble, with a solubility of 150 g in 100 ml of distilled water at 25°c. It has a slightly tarter taste than citric acid, with a tartness equivalent of 0.8–0.9. It augments the flavor of fruits in which it is a natural constituent. It is used in grapeand limeflavored beverages and grape-flavored jellies. It is used as an acidulant in baking powder and as a synergist with antioxidants to prevent rancidity.
Uses
D-(-)-Tartaric acid is commonly used as a resolving agent in organic synthesis. It is the synthetic enantiomer of L-(+)-Tartaric acid and is utilized in the production of synthetic analgesics. Tartaric acid is the second largest alpha hydroxy acid (AHA) in terms of size, with glycolic acid being the smallest and citric acid being the largest. It serves as a precursor for the synthesis of ester derivatives such as D-tartaric acid diethyl ester, D-tartaric acid dimethyl ester, and D-tartaric acid diiso-propyl ester. Moreover, it is employed in the creation of chiral aziridine derivative, which is a common intermediate for manufacturing hydroxyethylamine class HIV protease inhibitors like saquinavir, amprenavir, and nelfinavir. In the food industry, it is extensively used as a beer foaming agent, for regulating food acidity, and as a flavoring agent. However, due to its challenging workability and potential skin irritation, it is not frequently utilized in cosmetic or anti-aging preparations.
Definition
ChEBI: D-tartaric acid is the D-enantiomer of tartaric acid. It has a role as an Escherichia coli metabolite. It is a conjugate acid of a D-tartrate(1-). It is an enantiomer of a L-tartaric acid.
Biotechnological Production
Tartaric acid is generally produced from crude tartar and lees, which are byproducts of wine production. However, there are a few reports of fermentative production of tartaric acid by Gluconobacter suboxydans growing on Glucose or sorbitol. Vanadate plays a central role in this process. The microorganism forms 5-keto-D-gluconic acid, which is oxidized to tartaric acid. The vanadium catalyzes this reaction. Product concentrations up to 2.96 g.L-1 have been observed after 3 days of fermentation.
General Description
D-(-)-Tartaric acid is a polycrystalline solid, widely used as food additive. It has been reported to exhibit piezoelectric effect.
Purification Methods
Crystallise the acid from distilled H2O or *benzene/diethyl ether containing 5% of pet ether (b 60-80o) (1:1). Soxhlet extraction with diethyl ether has been used to remove an impurity absorbing at 265nm. It has also been crystallised from absolute EtOH/hexane and dried in a vacuum for 18hours [Kornblum & Wade J Org Chem 52 5301 1987]. [Beilstein 3 IV 1229.]
D-(-)-Tartaric Acid Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| DONBOO AMINO ACID COMPANY | +8613063595538 | donboo@donboo.com | China | 9363 | 58 |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 | admin@whrchem.com | China | 153 | 58 |
| Hebei Ganmiao New material Technology Co., LTD | +86-17332992504 +86-17332992504 | sales8@hbganmiao.com | China | 300 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 | admin@86-ss.com | China | 1001 | 58 |
| Runte International Trade Limited | 19565631292 19565631292 | lucky@sdruntechem.com | China | 270 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12834 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Shandong Huisheng Import & Export Co., Ltd. | +86-13176845580 +86-13176845580 | da@zhongda-biotech.com | China | 232 | 58 |
Related articles
- Innovative Uses and Eco-Friendly Production of D-Tartaric Acid in Sensor Technology and Bioengineering
- D-Tartaric acid boosts biosensor sensitivity for Vibrio parahaemolyticus, vital for food safety. Its eco-friendly biological s....
- Oct 23,2024
View Lastest Price from D-(-)-Tartaric Acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-23 | D-Tartaric acid
147-71-7
|
US $0.00 / KG | 1KG | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-12-23 | D-(-)-Tartaric Acid
147-71-7
|
US $999.00-666.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-12-23 | D-Tartaric acid
147-71-7
|
US $78.00-35.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- D-Tartaric acid
147-71-7
- US $0.00 / KG
- 99%
- Jinan Finer Chemical Co., Ltd
-

- D-(-)-Tartaric Acid
147-71-7
- US $999.00-666.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- D-Tartaric acid
147-71-7
- US $78.00-35.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd