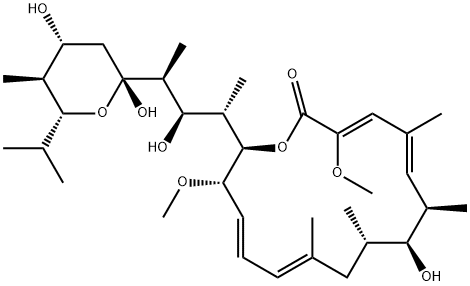
BAFILOMYCIN A1
- CAS No.
- 88899-55-2
- Chemical Name:
- BAFILOMYCIN A1
- Synonyms
- CS-1163;afilomycin A1;BAFILOMYCIN A1;Bafilomycin A1,95%;BAFILOMYCIN A1 95%;BAFILOMYCIN A1, 98+%;Bafilomycin A1(Baf-A1);BAFILOMYCIN A1 USP/EP/BP;BafilomycinA1fromStreptomycesgriseus;BAFILOMYCIN A1, STREPTOMYCES GRISEUS
- CBNumber:
- CB3743951
- Molecular Formula:
- C35H58O9
- Molecular Weight:
- 622.83
- MDL Number:
- MFCD06795130
- MOL File:
- 88899-55-2.mol
| Melting point | >106°C (dec.) |
|---|---|
| Boiling point | 582.86°C (rough estimate) |
| Density | 1.0594 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| RTECS | RN9781000 |
| Flash point | 87℃ |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 5 mg/ml, with warming). |
| pka | 12.66±0.70(Predicted) |
| form | Powder or Solid |
| color | White to off-white |
| Water Solubility | Soluble in methanol, ethanol, acetone and chloroform. Insoluble in water. |
| BRN | 4730700 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335-H227 | |||||||||
| Precautionary statements | P210e-P280a-P370+P378a-P403+P235-P501a-P261-P304+P340-P305+P351+P338-P405 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | 3172 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29419090 | |||||||||
| NFPA 704 |
|
BAFILOMYCIN A1 price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B1793 | Bafilomycin A1 from Streptomyces griseus ≥90% (HPLC) | 88899-55-2 | 2μg | $86.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML1661 | Bafilomycin A1 Ready Made Solution 0.16?mMinDMSO,fromStreptomycesgriseus | 88899-55-2 | 0.1ml | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | B1793 | Bafilomycin A1 from Streptomyces griseus ≥90% (HPLC) | 88899-55-2 | 10μg | $210 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.08409 | InSolution Bafilomycin A1, ≥97% by HPLC - CAS 88899-55-2 - Calbiochem A 100 μM (15 μg/241 μL) sterile-filtered solution of Bafilomycin A1, | 88899-55-2 | 15μG | $280 | 2024-03-01 | Buy |
| Sigma-Aldrich | 196000 | Bafilomycin A1, Streptomyces griseus - CAS 88899-55-2 - Calbiochem Bafilomycin A1, CAS 88899-55-2, acts as a highly potent and specific inhibitor of vacuolar-type H+-ATPase (Ki = 500 pM). Blocks the fusion of autophagosome with lysosome. | 88899-55-2 | 10μG | $201 | 2024-03-01 | Buy |
BAFILOMYCIN A1 Chemical Properties,Uses,Production
Description
Bafilomycin A1 is a fungal metabolite that has been found in Streptomyces and has diverse biological activities. It is an inhibitor of vacuolar H+-ATPases (V-ATPases; Ki = 0.5 nM in N. crassa vacuolar membranes) and is greater than 1,000-fold selective for V-ATPases over Na+/K+-, Ca2+-, and H+-ATPases. Bafilomycin A1 (100 nM) inhibits autophagosome maturation and protein degradation in H-4-II-E cells. It inhibits chloroquine-induced apoptosis in primary cerebellar granule neurons (CGNs) but not chloroquine-induced inhibition of macroautophagy. Bafilomycin A1 (100 nM) reduces viral yield in the culture supernatant of Vero E6 and Huh7 cells, as well as HEK293T cells expressing human angiotensin-converting enzyme 2 (ACE2), infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It also reduces lung RNA copy numbers and viral pneumonia in ACE2 transgenic mice infected with SARS-CoV-2 when administered at a dose of 0.1 mg/kg.
Chemical Properties
white to off-white powder
Uses
A macrolide antibiotic and potent and selective inhibitor of vacuolar-type (v-type) H+ ATPase
Uses
Bafilomycin A1 is a specific potent inhibitor of vacuolar ATPases.It is used as an antibacterial, antifungal, antineoplastic and an immunosuppressive. It prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes and is a macrolide antibiotic and potent and selective inhibitor of vacuolar-type H+ ATPase (V-ATPase).
Uses
Bafilomycin A1 is a member of a potent family of macrocyclic lactones with broad spectrum biological activity, including activity against bacteria, yeast, fungi, nematodes, insects and tumour cell lines. Bafilomycin A1 is an inhibitor of vacuolar-type ATPase.
Definition
ChEBI: The most used of the bafilomycins, a family of toxic macrolide antibiotics derived from Streptomyces griseus.
General Description
A macrolide antibiotic that acts as a specific inhibitor of vacuolar-type H+-ATPase (V-type; Ki = 500 pM). A valuable tool for distinguishing among different types of ATPases. Blocks lysosomal cholesterol trafficking in macrophages and is known to interfere with pH regulation in brain cells. Exhibits cytotoxic effects on a number of cell lines in a cell viability assay. Reported to selectively inhibit β-secretase, an enzyme involved in the processing of amyloid precursor protein (APP). The InSolution format with a purity of ≥97% by HPLC in 90% DMSO is also available (Cat. No. 508409).
Biological Activity
Highly potent, selective inhibitor of vacuolar H + -ATPases (IC 50 = 500 pM as measured in chromaffin granule membranes). Selective over other ATP hydrolyzing enzymes such as F-ATPases, Ca 2+ -ATPases, Na + /K + -ATPases and plasma membrane H + -ATPases.
Biochem/physiol Actions
Bafilomycin A1 is a macrolide antibiotic. Bafilomycin A1 acts as a potent and selective inhibitor of vacuolar-type H+-ATPase.
storage
-20°C
References
1) Werner?et al.? (1984) Metabolic products of microorganisms. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity; J. Antibiot.,?37?110 2) Drose and Altendorf? (1997)?Bafilomycins and concanamycins as inhibitors of V-ATPase and P-ATPase;?J. Exp. Biol.,?200?1 3) Yamamoto?et al. (1998)?Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells;?Cell Struct. Func.,?23?33
BAFILOMYCIN A1 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9218 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32072 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 23125 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9640 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19908 | 58 |
View Lastest Price from BAFILOMYCIN A1 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-07 | Bafilomycin A1
88899-55-2
|
US $699.00-287.00 / mg | 99.14% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-07-17 | BAFILOMYCIN A1 USP/EP/BP
88899-55-2
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited | |
 |
2019-07-06 | BAFILOMYCIN A1
88899-55-2
|
US $1.00 / kg | 1kg | 95%-99% | 100kg | Career Henan Chemical Co |
-

- Bafilomycin A1
88899-55-2
- US $699.00-287.00 / mg
- 99.14%
- TargetMol Chemicals Inc.
-

- BAFILOMYCIN A1 USP/EP/BP
88899-55-2
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- BAFILOMYCIN A1
88899-55-2
- US $1.00 / kg
- 95%-99%
- Career Henan Chemical Co